About Phentermine
What do Phentermine capsules or tablets do?
Phentermine is an appetite suppressant used for the short-term treatment of obesity. When used in conjunction with diet, exercise, and behavior therapy, Phentermine may help you to lose weight while you are learning new ways to eat and to exercise.
How do you know which dosage to start off with?
It is best to use this drug under a doctor’s care. The average dose is 30mgs/day. If you are a new user, buy a low number of pills as a test, and start with the lowest dosage.
Does Phentermine work for long term use, and how long should I take the medication for?
Phentermine is approved for the short-term treatment (6-12 weeks) of obesity. To continue losing weight and prevent its return, you must develop and continue on a long-term basis - good dietary, exercise, and behavioral habits.
Few studies have been conducted concerning the long-term use of Phentermine. The National Institute of Health (NIH) has conducted a comparison of long-term studies of diet medications.
How long will Phentermine remain in my body for after I stop taking it?
Currently, no clinical study is available that measures the length of time that Phentermine will stay in your body after you stop treatment.
If taking it for a long period of time, will it cause problems to some of my major organs?
Phentermine is prescribed for short term treatment of obesity; therefore there are no clinical findings of the long term effects of this drug.
What are the active ingredients in the medications?
Phentermine Hydrochloride.
Does Phentermine contain Ephedra?
No, the only active ingredient is Phentermine Hydrochloride. Ephedra is a completely different chemical substance.
What side effects should I expect that are normal within the first week of using the medication?
Blurred vision, dry mouth, sleeplessness, irritability, stomach upset or constipation may occur the first few days as your body adjusts to the medication. The main advantage of phentermine is that it suppresses the appetite; however, the drug also elevates the heart rate and blood pressure.
Is this medication better than using, i.e., hydroxycut, metabolife, and body solutions, and if so, why?
This really depends on your medical history, and how your body reacts to the different medications mentioned, and if the medication is most suited to your needs. Phentermine should NOT be taken if you have any of the following conditions: heart disease, high blood pressure, glaucoma, thyroid problems, anxiety disorder, diabetes, epilepsy or any other seizure disorder. Do not take Phentermine if you are taking monoamine oxidase inhibitors (MAOI) to treat depression. Also, if you are pregnant or nursing, talk to your doctor about the potential risks involved with taking Phentermine.
The success of Phentermine as a weight loss drug is probably due to the fact that it is one of the oldest FDA approved weight loss drugs in the market, and because it is cheaper than other diet pills.
Who should use Phentermine?
Phentermine is recommended for people that have a body mass index (BMI) of 28 or more. It is only intended for those with major weight problems.
What is the difference between the yellow and the blue Phentermine capsules?
The yellow phentermine 30mg capsules and the blue Phentermine 30 mg capsules act the same. Blue Phentermine and Yellow Phentermine both have the same active ingredient (i.e. Phentermine Hydrochloride).
Both of these capsules are manufactured by Eon Labs. The blue/white version is a generic for Fastin and the yellow is just a generic phentermine. The ingredients are the same. The only real differences are the aspect (color and powder vs. little pellets) and the price.
Please note that the yellow capsules are not a generic equivalent for Ionamin. There is no generic equivalent for Ionamin, which is the time released resin form of Phentermine.
As for the 15 mg capsules, they are simply a lower dosage form of Phentermine that is manufactured by Eon Labs.
The 37.5 mg Phentermine is a generic for Adipex-P that is manufactured by Purepac. The only difference between the brand name (Adipex-P, manufactured by Gate Pharma) and the generic is price.
For a more technical explanation, please contact the manufacturers Eon Labs and Purepac.
What is Fastin?
Fastin was the brand name of Phentermine produced by King Pharmaceuticals for SmithKline Beecham. In December 1998, SK-Beecham withdrew Fastin from the market. Since Fastin is no longer produced and sold, it is not included in our Pictures of Phentermine section.
What is Adipex?
Adipex is the brand name of Phentermine produced by Gate Pharmaceuticals.
What is Ionamin?
Ionamin is the brand name of Phentermine marketed by Medeva Pharmaceuticals. Ionamin capsules use a special delivery system, known as resin matrix, to deliver Phentermine. Although there are other Phentermine products on the market, none has this resin matrix.
What’s the difference between Ionamin and Adipex?
Ionamin is the timed-release resin form of Phentermine and Adipex is the brand name for Phentermine Hydrochloride. Ionamin’s timed-release form means that this tablet lasts longer, but with a milder effect, while Adipex is said to last 10-12 hours. However, the differences between these two are subjective.
May I take Phentermine and Xenical at the same time?
No official clinical study has been conducted on the use of Phentermine in conjunction with Xenical. Prior to taking any prescription medicine, please inform your doctor or healthcare professional of your complete medical history, including any other medicines you may be taking (both prescription and non-prescription). Based on your medical history and any medications you are currently taking, your doctor will determine if you may safely take Phentermine and Xenical.
What is Phen-Pro?
“Phen-Pro” is the combination (cocktail) of Phentermine (the “Phen”) and a low dosage of Prozac (the “Pro”). Any one of the following antidepressants: Zoloft, Celexa, Luvox, Trazadone or Effexor may be used in lieu of Prozac.
The use of the antidepressant in the Phen-Pro cocktail is unrelated to depression. The cocktail is necessary because the effects of Phentermine, when used alone, lessen over time. The Phen-Pro cocktail enables Phentermine to work better and for a longer period of time.
Phen-Pro is considered an “off-label” use, meaning that the FDA, who often voice concerns regarding the mixing/combining of medications, does not approve it. However, once the agency has approved a drug, doctors may prescribe it at will.
The combination of Phentermine and Prozac does not appear to cause the problems that resulted from the usage of Fen-Phen.
Is Phentermine an Amphetamine?
No, Phentermine is not an Amphetamine. Phentermine is, however, similar chemically to the Amphetamines. In the diagram below, we have reproduced the molecular structures of Phentermine, Methamphetamine and Amphetamine.
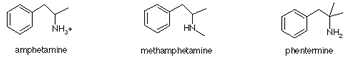
The three molecules are chemically similar, but are not identical.
Will Phentermine show as a positive result to a urine drug test?
As Phentermine is similar chemically to Amphetamines, it may cause a positive result in urine screening tests for Amphetamines.
What is Fen-Phen (or Phen-Fen)?
Fen-Phen is the combination of Fenfluramine or Pondimin (the “Fen”) and Phentermine (the “Phen”). Fenfluramine and Phentermine are prescription medications used as appetite suppressants for the short-term (a few weeks) management of obesity.
Phentermine received FDA approval in 1959 and Fenfluramine in 1973. Together, the two medications produced a powerful diet drug. The FDA had never approved the Fen-Phen combination, but once the agency has approved a drug, doctors may prescribe it at will.
Phentermine has also been used in combination with Dexfenfluramine (“Dexfen-Phen”). Dexfenfluramine (or Redux) was approved in 1996 for use as an appetite suppressant in the management of obesity.
In 1992, Dr. Michael Weintraub of the University of Rochester and several colleagues published a study showing Fen-Phen as far more effective than dieting or exercise in reducing the weight of the chronically obese. Initially, Fen-Phen seemed to be without immediate side effects.
Soon, Fen-Phen was on the market. In just a short time, Fen-Phen became a national sensation, with 6.6 million prescriptions in 1996.
Dexfen-Phen - The combination of Redux or Dexfenfluramine - a more refined compound that, like Fen-Phen, affects seratonin levels, but with fewer side effects than Fenfluramine - and Phentermine also became a sensation.
Unfortunately, neither combination was tested for safety… By the summer of 1997, the Food and Drug Administration (FDA) found 24 cases of heart-valve deterioration in women who had taken the Fen-Phen combination. Throughout the summer, the FDA received additional reports of heart disease, including reports from patients who had taken only Fenfluramine or Dexfenfluramine.
In September 1997, The FDA requested drug manufacturers to voluntarily withdraw Fenfluramine and Dexfenfluramine. The FDA also recommended that patients using either Fenfluramine or Dexfenfluramine stop doing so.
The FDA did NOT, however, request the withdrawal of the third drug involved in the cocktails, Phentermine.
Revision date: June 18, 2011
Last revised: by Andrew G. Epstein, M.D.