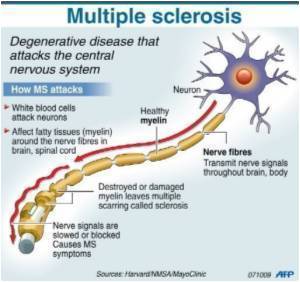
Cannabis constituent has no effect on MS progression, study shows
The first large non-commercial clinical study to investigate whether the main active constituent of cannabis (tetrahydrocannabinol or THC) is effective in slowing the course of progressive multiple sclerosis (MS), shows that there is no evidence to suggest this; although benefits were noted for those at the lower end of the disability scale.
The study is published in The Lancet Neurology.
The CUPID (Cannabinoid Use in Progressive Inflammatory brain Disease) study was carried out by researchers from Plymouth University Peninsula Schools of Medicine and Dentistry. The study was funded by the Medical Research Council (MRC), the Multiple Sclerosis Society and the Multiple Sclerosis Trust, and managed by the National Institute for Health Research (NIHR) on behalf of the MRC-NIHR partnership.
CUPID enrolled nearly 500 people with MS from 27 centres around the UK, and has taken eight years to complete. People with progressive MS were randomised to receive either THC capsules or identical placebo capsules for three years, and were carefully followed to see how their MS changed over this period. The two main outcomes of the trial were a disability scale administered by neurologists (the Expanded Disability Status Scale), and a patient report scale of the impact of MS on people with the condition (the Multiple Sclerosis Impact Scale 29).
Overall the study found no evidence to support an effect of THC on MS progression in either of the main outcomes. However, there was some evidence to suggest a beneficial effect in participants who were at the lower end of the disability scale at the time of enrolment but, as the benefit was only found in a small group of people rather than the whole population, further studies will be needed to assess the robustness of this finding.
One of the other findings of the trial was that MS in the study population as a whole progressed slowly, more slowly than expected. This makes it more challenging to find a treatment effect when the aim of the treatment is to slow progression.
 As well as evaluating the potential neuroprotective effects and safety of THC over the long-term, one of the aims of the CUPID study was to improve the way that clinical trial research is done, by exploring newer methods of measuring MS and using the latest statistical methods to make the most of every piece of information collected. This analysis continued for several months and has provided important information about conducting further large scale clinical trials in MS.
As well as evaluating the potential neuroprotective effects and safety of THC over the long-term, one of the aims of the CUPID study was to improve the way that clinical trial research is done, by exploring newer methods of measuring MS and using the latest statistical methods to make the most of every piece of information collected. This analysis continued for several months and has provided important information about conducting further large scale clinical trials in MS.
Professor John Zajicek, Professor of Clinical Neuroscience at Plymouth University Peninsula Schools of Medicine and Dentistry, said: “To put this study into context: current treatments for MS are limited, either being targeted at the immune system in the early stages of the disease or aimed at easing specific symptoms such as muscle spasms, fatigue or bladder problems. At present there is no treatment available to slow MS when it becomes progressive. Progression of MS is thought to be due to death of nerve cells, and researchers around the world are desperately searching for treatments that may be ‘neuroprotective’. Laboratory experiments have suggested that certain cannabis derivatives may be neuroprotective.”
 He added: “Overall our research has not supported laboratory based findings and shown that, although there is a suggestion of benefit to those at the lower end of the disability scale when they joined CUPID, there is little evidence to suggest that THC has a long term impact on the slowing of progressive MS.”
He added: “Overall our research has not supported laboratory based findings and shown that, although there is a suggestion of benefit to those at the lower end of the disability scale when they joined CUPID, there is little evidence to suggest that THC has a long term impact on the slowing of progressive MS.”
###
Andrew Gould
.(JavaScript must be enabled to view this email address)
University of Plymouth