Gene identified for spread of deadly melanoma
Researchers at Washington University School of Medicine in St. Louis have identified a gene linked to the spread of eye melanoma.
Although more research is needed, the researchers say the discovery is an important step in understanding why some tumors spread (metastasize) and others don’t. They believe the findings could lead to more effective treatments.
Reporting online in the journal Science Express, the team found mutations in a gene called BAP1 in 84 percent of the metastatic eye tumors they studied. In contrast, the mutation was rare in tumors that did not metastasize.
Metastasis is the most common cause of death in cancer patients, yet little is known about how cancer cells evolve the ability to spread to other parts of the body. There is growing evidence that mutations in so-called metastasis suppressor genes may promote the spread of cancer, while having little to do with earlier stages in the life of a tumor. Very few such genes have been identified, but this finding strongly implicates BAP1 as a new member of that small group.
“Scientists and physicians have been waiting for a rational, therapeutic target that we could use to treat high-risk patients,” says first author and Washington University ophthalmologist J. William Harbour, MD. “We believe this discovery may provide insights needed to hasten the development of therapies for these patients.”
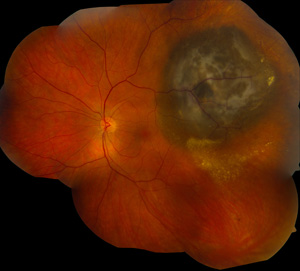 A cancerous melanoma tumor (dark area, upper right) is seen below the retina.
A cancerous melanoma tumor (dark area, upper right) is seen below the retina.
Harbour laboratory
Ocular melanoma, also called uveal melanoma, is the most common eye cancer and the second-most common form of melanoma, striking about 2,000 adults in the United States each year. It can affect people at any age but is most common in patients over 50. The tumors arise from pigment cells, called melanocytes, that reside in the layer below the retina called the uveal tract. Up to half of those with the cancer eventually develop metastatic disease, which is universally fatal.
“The most common site where the cancer spreads is the liver,” Harbour says. “If it spreads, it goes to the liver about 90 percent of the time, generally leading to death within months.”
To improve survival, scientists need to understand more about what causes the tumor cells to metastasize, according to Harbour, the Paul A. Cibis Distinguished Professor of Ophthalmology and Visual Sciences, professor of cell biology and of molecular oncology and director of ocular oncology at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine.
Harbour and co-investigator Anne M. Bowcock, PhD, professor of genetics of pediatrics and of medicine, have been looking at DNA in tumor cells for clues about why some tumors spread. Tumors had already been grouped into two classes based on gene expression profiles. Class 1 tumors have a low risk of spreading, while class 2 tumors carry a high risk of metastasis. In addition, 90 percent of class 2 tumors have lost a copy of chromosome 3, unlike class 1 tumors, which tend to retain both copies of the chromosome.
In this study, the team looked for differences in genes on chromosome 3 between the cells in class 1 and class 2 tumors. They started with tissue taken from a pair of class 2 tumors.
 Bowcock
Bowcock
“We looked for common genetic differences, called polymorphisms, that would unlikely have much of an effect,” Bowcock says. “We eliminated those variations and then went back to look at which gene on chromosome 3 had additional alterations. There was one gene, called BAP1, that had mutations in both of the tumors we analyzed.”
BAP1 is short for BRCA1-associated protein 1. As it happens, BRCA1 is linked to breast cancer in some women.
“It points, possibly, to a common theme in cancer genetics,” Bowcock says. “After identifying mutations in BAP1 in the first two tumors, we went back and looked at DNA from another 29 class 2 tumors, as well as 25 class 1 tumors. And we found that 84 percent of the class 2 tumors had damaging mutations in BAP1. We also found that in most cases, the class 2 tumor cells had only one copy of chromosome 3 – where the gene is located – so patients had only a single copy of the BAP1 gene, and because of damaging mutations, it could not fulfill its proper role in the cell.”
It appears that what the gene is supposed to do, Harbour says, is to act as a metastasis suppressor. When it is damaged, the tumor can spread.
“There are several ways this discovery could improve patient care,” Harbour says. “If we could detect BAP1 mutations at an earlier stage, for example, we might be able to monitor a patient’s blood for detectable melanoma cells as an early sign that they’re developing metastatic disease.”
He also says a better understanding of the normal role of the BAP1 protein could provide powerful insights into ways to therapeutically target eye tumors that are likely to spread. He and Bowcock already are beginning those studies.
“We know now that BAP1 is the big player in class 2 tumors, but there are other players, too,” Bowcock adds. “We’d also like to understand what other genes are mutated in class 1 tumors and why they don’t metastasize.”
Bowcock and Harbour identified a single class 1 tumor with a BAP1 mutation. One possible explanation for that finding may be that the tumor was evolving into a class 2 tumor.
In a second series of experiments, the researchers found that if they put ocular melanoma cells in culture and depleted their supply of BAP1, the cells began to change in appearance and to resemble class 2 tumors in just five days.
“Now we’re trying to knock down BAP1 levels for weeks to months and find out whether we start to see some of the chromosomal changes that are present in class 2 tumors,” Harbour says.
He says it remains unclear whether class 1 and class 2 tumors are different from their very inception or whether ocular melanoma tumors begin their existence as class 1 tumors and then, eventually, develop cells with BAP1 mutations.
“We have hints, both from experimental work and from patient samples, that the latter scenario is more likely, that the tumors start off as class 1 and evolve into class 2 tumors,” Harbour explains. “But that is still somewhat speculative, and we’ll need to do more experiments to test that hypothesis.”
The BAP1 mutation represents only the second common genetic mutation ever reported in ocular melanoma, and it is the only mutation linked to metastasis in this type of cancer.
“This finding will fundamentally alter the concepts and methodologies employed in patient management and in research in this field,” Bowcock says. “For example, it should lead to new diagnostic tests to distinguish benign from malignant growths of the eye, which could avoid thousands of needless, vision-threatening treatments each year while allowing earlier interventions in the few patients who truly harbor a malignant melanoma. In addition, the insights gained from this research into how BAP1 functions at a molecular level might pave the way for innovative new therapeutic approaches to the previously recalcitrant problem of metastatic disease.”
###
Harbour JW, Onken MD, Roberson EDO, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanoma. Science Express, science.1194472
This research was supported by grants from the National Cancer Institute, National Eye Institute, National Institutes of Health, Barnes-Jewish Hospital Foundation, Kling Family Foundation, Tumori Foundation, Horncrest Foundation and Research to Prevent Blindness.
J. William Harbour, MD, and Washington University may receive future income based on the university’s licensing of a related technology to Castle Biosciences Inc.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked fourth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
Siteman Cancer Center is the only NCI-designated Comprehensive Cancer Center within a 240-mile radius of St. Louis. Siteman Cancer Center is composed of the combined cancer research and treatment programs of Barnes-Jewish Hospital and Washington University School of Medicine.
###
By Jim Dryden