New Treatment for Age Related Macular Degeneration
Vanderbilt University Medical Center recently began offering a new treatment for wet age-related macular degeneration (AMD) that may improve, and in some cases restore, patients’ vision.
Lucentis, also known by the generic name ranibizumab, was approved by the FDA last month. The drug is administered by injection directly into the eyeball of patients suffering from wet AMD, a chronic condition caused by abnormal growth of blood vessels behind the eye. The disorder leads to leaking and/or bleeding within the eye, causing central vision loss and often blindness.
Nearly 6 million Americans 55 and older suffer from AMD, with 1.5 million suffering from some vision loss. It is estimated that 100,000 people develop wet AMD each year.
This is the first drug to show promise of significantly improving visual acuity for these patients, said Paul Sternberg, M.D., director of the Vanderbilt Eye Institute. While other treatments slowed the progression of the disease, Lucentis helped improve vision by inhibiting the growth of blood vessels.
“This represents a tremendous step forward for patients with wet AMD,” said Sternberg. “In the past we have told them that there is nothing we can do. Now, we are hopeful and excited that we can tell them there are drugs that might be able to help slow down the deterioration.” 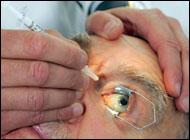
“We now can offer a drug that has the potential to improve visual function,” added Franco Recchia, M.D., who was the first to administer the drug at Vanderbilt.
And that’s just what Boggs Huff, 81, is hoping for.
Huff was diagnosed in 2000 with the beginning stages of AMD in both eyes. Last year he underwent cataract and corneal replacement surgery in his left eye, which was showing signs of worsening.
“Doing the surgeries worked great,” said Huff. “It was amazing, it changed my sight. I could read without a magnifying glass and I could see the signs on the road.”
But it was short-lived. Three months later he developed wet AMD and came to Sternberg in early 2006.
“I feel hopeful,” Huff said. “I live in hope, but I am prepared to know that if it doesn’t help, I tried it all. When you start losing your eyesight, you’ll go through any route. You are more aggressive about finding out what is available to help you. It sure gets your attention.”
In clinical trials of Lucentis, close to 40 percent of patients experienced three or more lines of vision improvement.
Sternberg cautions that the treatments come at a cost. Side effects can include an intraocular infection called endophthalmitis, retinal detachment and thromboembolic events. Each injection, 0.5 milligrams/cc, costs nearly $2,000. Many insurance companies have covered the treatment, Sternberg said.
Sternberg and his team, which includes Anita Agarwal, M.D., have treated about 10 patients thus far. All have tolerated the treatments without complication, Sternberg said. Each patient will return for at least three more doses for a total of four treatments, at which time they will be evaluated for visual acuity.
Sternberg is George W. Hale Professor and Chair of Ophthalmology and Visual Sciences.
Vanderbilt University Medical Center
Revision date: July 7, 2011
Last revised: by Sebastian Scheller, MD, ScD