Optimizing the Antihormonal Treatment and Prevention of Breast Cancer
The treatment for advanced breast cancer has changed dramatically over the past three decades.
Initially, advanced breast cancer therapeutics focused on nonspecific cytotoxic agents. The reinvention of tamoxifen from a failed “morning after pill” to the first targeted therapy for breast cancer provided the clinical research community with an invaluable new therapeutic tool to pioneer the strategy of long term antihormonal therapy and chemoprevention27). Not surprisingly, enthusiasm from the medical community and pharmaceutical industry was not high. The early trials have been summarized with approximately a 30% response rate in affected patients28). This improves if ER positive patients are selected for targeted treatment. Tamoxifen responses were the same as any other endocrine approach to breast cancer; however, the advantage of tamoxifen was the low incidence of side effects compared to other endocrine therapies29).
The use of tamoxifen as the pioneering agent as an adjuvant to surgery has its origins in studies from the 1970’s27). The laboratory studies demonstrated the feasibility of targeting the ER and using long term anti-hormonal therapy30, 31) so that tamoxifen could be reasonably considered for use as an adjunct to surgery in node positive and then node negative patients. The reason for using tamoxifen as an adjuvant following surgery is to prevent recurrence. The clinical trials ultimately showed both increases in disease-free and overall survival with adjuvant tamoxifen treatment21). The studies demonstrated that there is a 50% decrease in recurrence in ER positive patients with 5 years of tamoxifen. Even 15 years after a diagnosis of ER positive breast cancer, treatment with 5 years of tamoxifen continues to decrease mortality32).
However, there is no increase in disease free or overall survival with ER negative cancer.
The transition of tamoxifen from a short term treatment to a long term therapy for node positive and node negative breast cancer increased awareness of the pharmacology and side effects of tamoxifen33). Tamoxifen is a SERM, so the drug preserves bone density and potentially reduces the risk of fractures secondary to its agonist effects on the ER receptors in bone. Additionally, tamoxifen decreases circulating cholesterol, but this side effect is thought to greatly improve patient prognosis. The negative side effects include an increase in the incidence of endometrial cancer and venous thrombosis. However, the benefits of tamoxifen treatment greatly outweigh the risks of endometrial cancer and venous thrombosis32).
The ubiquitous use of tamoxifen in the treatment plan for breast cancer has improved patient prognosis and enhanced survivorship dramatically. Nevertheless, the knowledge of the estrogen-like side effects of tamoxifen has focused research efforts on understanding tamoxifen induced drug resistance and the development of new and safer agents to treat breast cancer.
Drug resistance to Tamoxifen:
Resistance to therapy is most often observed during the treatment of advanced breast cancer.
After 1-3 years of tamoxifen treatment, breast tumors start to grow despite continuing tamoxifen therapy. However, what is unique about tamoxifen resistant tumors is that a withdrawal response occurs if tamoxifen treatment is stopped34). The tumor is dependent on tamoxifen. This phenomenon is best illustrated in the laboratory. Animal models have demonstrated that ER + breast cancer lines can eventually develop resistance to tamoxifen and the tumors then grow in response to tamoxifen35). The resistance to tamoxifen is believed to occur because of an increase in cell surface signaling through the HER2/neu/EGFR or Insulin like growth factor receptors that promote phosphorylation of the ER and its coactivators36). This in turn activates breast cancer cell growth (Fig 2). Based on the recognition that tamoxifen has limitations and that less estrogenic like drugs would be useful therapeutic agents, it is reasonable to examine the rational application of aromatase inhibitors and fulvestrant.
Second Line Therapy Following the Failure of Tamoxifen
In addition to stopping tamoxifen, there are multiple ways to address failure of tamoxifen therapy and subsequent resistance to tamoxifen (Fig 3). At present, the first two options are hypothetical. Clinicians have an intense interest in the feasibility of blocking the cell surface receptors with monoclonal antibodies or blocking the tyrosine kinases that cause tamoxifen resistance.
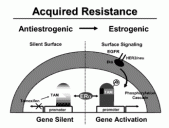 Fig 2. The proposed mechanism of tamoxifen resistance. With a silent surface, tamoxifen successfully prevents ER stimulated proliferation by altering the shape of the ER. With surface signaling, an increase in cell surface signaling through the HER2/neu/EGFR receptor promotes phosphorylation of the ER. This leads to proliferation of breast cancer cells in the presence of tamoxifen or estrogen.
Fig 2. The proposed mechanism of tamoxifen resistance. With a silent surface, tamoxifen successfully prevents ER stimulated proliferation by altering the shape of the ER. With surface signaling, an increase in cell surface signaling through the HER2/neu/EGFR receptor promotes phosphorylation of the ER. This leads to proliferation of breast cancer cells in the presence of tamoxifen or estrogen.
At present there are suggestions that blocking the HER2/neu pathway has promise, but only in patients with gene amplification. In contrast, the second two options for second line therapy are based on the mechanism of tamoxifen resistance. Laboratory studies show that tamoxifen resistant tumors will grow both with tamoxifen and estrogen35). Estrogen is produced by aromatization in postmenopausal women. Therefore, a plan to stop the reactivation of tumor growth after tamoxifen is reasonable.
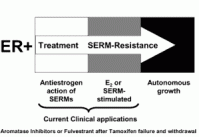
Aromatase inhibitors are the agent of choice to create a “no estrogen” state37, 38). Fulvestrant can also be used to destroy the estrogen receptor completely. In the absence of the ER, estrogen stimulated proliferation cannot occur, regardless of the cellular signaling mechanisms that activate the ER. Clinical studies of second line therapies after the development of tamoxifen resistance show that anastrazole and fulvestrant are equally effective in controlling breast tumor growth39, 40).
Thus, for the purposes of clinical clarity, the treatment paradigm for patients who fail SERM therapy can be summarized as shown in figure 4.
Adjuvant Therapy with Aromatase Inhibitors
Since aromatase inhibitors do not have the estrogen-like side effects noted with tamoxifen and there is clinical evidence that they can be used once resistance to tamoxifen occurs, the logical question arises of whether they are an improvement over tamoxifen in the clinical setting.
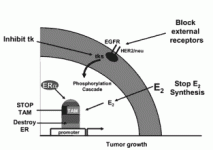 Fig 3. Possible strategies to prevent ER receptor activation in SERM resistant breast cancer. Herceptin, a monoclonal antibody blocks Her2/neu/EGFR to prevent phosphorylation of the ER. Alternatively, a number of small molecules that inhibit the receptor tyrosine kinase are being explored. Aromatase inhibitors prevent the formation of estrogen in a postmenopausal woman’s body. Without estrogen available to bind the receptor, the tamoxifen resistant cells cannot proliferate. Another option is to destroy the receptor completely with fulvestrant, a pure antiestrogen that binds to the ER. The strange shape of the complex programs the ER complex for rapid destruction. Thus, there will not be any ER mediated replication of cancer cells.
Fig 3. Possible strategies to prevent ER receptor activation in SERM resistant breast cancer. Herceptin, a monoclonal antibody blocks Her2/neu/EGFR to prevent phosphorylation of the ER. Alternatively, a number of small molecules that inhibit the receptor tyrosine kinase are being explored. Aromatase inhibitors prevent the formation of estrogen in a postmenopausal woman’s body. Without estrogen available to bind the receptor, the tamoxifen resistant cells cannot proliferate. Another option is to destroy the receptor completely with fulvestrant, a pure antiestrogen that binds to the ER. The strange shape of the complex programs the ER complex for rapid destruction. Thus, there will not be any ER mediated replication of cancer cells.
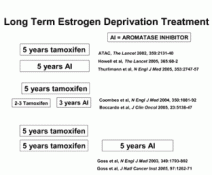
Five different studies (Fig 5) have shown that the aromatase inhibitors, anastrazole, letrozole, and exemestane are better than tamoxifen at preventing contralateral breast cancer, improving disease-free survival, decreasing the risk of endometrial cancer, and have no additional risk of blood clots. In all of the studies, the patient demo-graphics, stages of cancer, and hormone receptor status were well matched between control and experimental groups. The primary endpoint was locoregional and distant recurrences. Disease-free survival was compared between treatment and control groups as well as side effect profiles, adverse events, and deaths related or unrelated to breast cancer.
Several questions were addressed by the different studies. One question that was addressed by Boccardo et al.41) was whether or not switching to anastrazole after two to three years of tamoxifen would help prevent relapse. The median follow up time was 36 months. One group received tamoxifen for 5 years (225 patients) and the other group received tamoxifen for 2-3 years followed by anastrazole for 3 years (223 patients). Patients who switched to anastrazole had a longer disease free survival. The difference between switching to anastrazole and continuing tamoxifen was 5.8%41).
 In addition, another larger international study examined the use of exemestane after 2-3 years of tamoxifen (2380 patients) vs. continuing tamoxifen (2362 patients), The design was similar to the study design by Boccardo et al. and the disease free survival was improved by 4.7 % when patients were switched to exemestane42).
In addition, another larger international study examined the use of exemestane after 2-3 years of tamoxifen (2380 patients) vs. continuing tamoxifen (2362 patients), The design was similar to the study design by Boccardo et al. and the disease free survival was improved by 4.7 % when patients were switched to exemestane42).
However, the question has been asked “can an aromatase inhibitor improve prognosis after the full five years of tamoxifen treatment?” Goss and his coworkers have compared letrozole (2575 patients) and placebo (2582 patients) after 5 years of tamoxifen in breast cancer survivors. The median follow up was 2.4 years and the disease free survival was 93% in the letrozole group and 87% in the placebo group, with an absolute difference of 6%43).
Since there are benefits to switching to aromatase inhibitors, why not use an aromatase inhibitor immediately following surgery? This next question was addressed by two large multinational clinical trials. The ATAC trial compared anastrazole, tamoxifen or a combination of tamoxifen and anastrazole to see if disease free survival improved44). There were over 9000 patients enrolled to receive tamoxifen alone (3116 patients), anastrazole alone (3125 patients), or anastrazole plus tamoxifen (3125 patients). The disease free survival after 3 years of anastrazole, tamoxifen, or a combination of the two was 89.4%, 87.4%, and 87.2%. Additionally, after 3 years, hazard ratios favored anastrazole over tamoxifen for node negative and node positive disease (0.85 for node positive and 0.7 for node negative)44).
 Since there was no difference between the combination arm and the tamoxifen arm, the combination arm was closed and the anastrazole (n=2618) and tamoxifen patients (n=2598) were followed further for 2.7 years. While overall survival was the same between the two groups, the disease free survival (absolute difference of 3%), time to recurrence (absolute difference of 3.7%), and time to distant recurrence (absolute difference of 2%), were better in the group that received anastrazole vs. tamoxifen. Patients who received anastrazole (n=3092) instead of tamoxifen (n=3094) had a decrease in the risk of contralateral breast cancer (35 vs. 59 patients). In addition, the patients who took anastrazole also had a decrease in endometrial cancer (5 vs. 17 patients) and blood clots (87 vs. 140 patients)45).
Since there was no difference between the combination arm and the tamoxifen arm, the combination arm was closed and the anastrazole (n=2618) and tamoxifen patients (n=2598) were followed further for 2.7 years. While overall survival was the same between the two groups, the disease free survival (absolute difference of 3%), time to recurrence (absolute difference of 3.7%), and time to distant recurrence (absolute difference of 2%), were better in the group that received anastrazole vs. tamoxifen. Patients who received anastrazole (n=3092) instead of tamoxifen (n=3094) had a decrease in the risk of contralateral breast cancer (35 vs. 59 patients). In addition, the patients who took anastrazole also had a decrease in endometrial cancer (5 vs. 17 patients) and blood clots (87 vs. 140 patients)45).
The initial portion of the BIG study addressed the use of letrozole (n=4003) in comparison with tamoxifen (n=4007)46). An additional study will compare letrozole followed by tamoxifen, and tamoxifen followed by letrozole. At five years, patients taking letrozole had less recurrence than those on tamoxifen as demonstrated by an absolute difference of 3.4%. In the letrozole group, 16 patients developed a contralateral breast cancer, while 27 patients in the tamoxifen group developed a contralateral breast cancer. Most importantly, the BIG trial further stratified patients into node negative and node positive groups as far as disease free survival rates. When looking at disease-free survival alone, the patients with node positive cancer benefited the most from letrozole (hazard ratio= .71). The 5 year disease-free survival was 77.9% in those patients who received letrozole vs. 71.4% in those who received tamoxifen. Patients with node negative cancer had an 88.7% rate of disease-free survival (hazard ratio of 0.96) regardless of the use of letrozole or tamoxifen. Fewer women had endometrial cancer in the letrozole group (4/3089 patients) when compared to those patients taking tamoxifen (10/3157 patients). In addition, the patients taking letrozole had a significant decrease in thromboembolic events (61/3975 patients) in comparison to the patients who received tamoxifen (140/3988)46).
Overall, the trials have shown that aromatase inhibitors are favorable to tamoxifen in postmenopausal, ER positive patients, secondary to decreased recurrence and a decrease in undesirable side effects such as endometrial cancer and venous thrombosis. The concern with aromatase inhibitors is the higher rate of osteoporosis and subsequent fractures as well as higher serum cholesterol levels. Practitioners must be proactive and monitor patients for joint pain and do regular cardiovascular risk stratification. Fortunately, there are medicines such as statins to lower cholesterol and bisphosphonates to maintain bone density that can now be a part of the patient’s treatment plan.