Breast cancer vaccine shows promise in study
Houston researchers on Wednesday reported positive results with an experimental breast cancer vaccine, a promising development in an emerging field in cancer care.
The vaccine, one of many now in testing, significantly decreased the recurrence rate of breast cancer in women who had been treated for a common tumor type, according to a study led by the University of Texas M.D. Anderson Cancer Center.
“If this vaccine and others continue to perform well in trials, they could change practice standards in breast cancer,” said Dr. Elizabeth Mittendorf, an M.D. Anderson surgical oncology professor and the study’s principal investigator.
“That’s not to say it will take the place of surgery, chemotherapy or radiation, but it could became incorporated into standard care.”
Mittendorf said cancer researchers are in “the dawn of a new era,” in which scientists are learning to manipulate the immune system to recognize cancer cells and prevent or treat the disease. She said vaccines for breast cancer have lagged behind those for other cancer types but are starting to catch up.
The new vaccine triggers the patient’s immunity against a tumor protein, known as HER2, present to some extent in 75 percent to 80 percent of breast cancers.
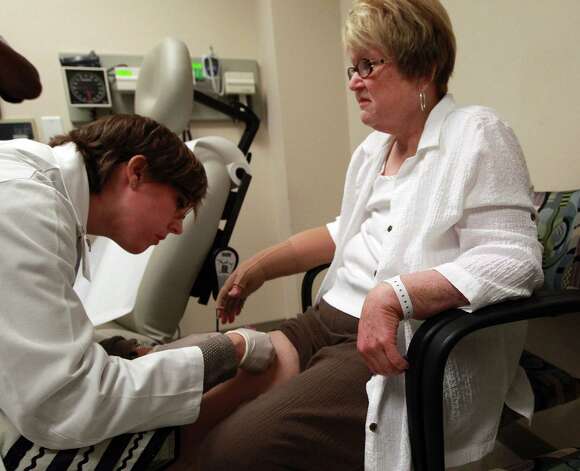 Sara Stassen, RN, left, at The University of Texas MD Anderson Cancer Center inoculates patient Patricia Anne Allan, 67, of Topeka, Kan., with the AE37 breast cancer vaccine that has show to elicit an immune response in women with varying levels of the human epidermal growth factor receptor2 (HER2) a protein that aids in the progression of certain aggressive types of breast cancer Wednesday, May 16, 2012, in Houston. “The vaccine educates the immune system to recognize HER2 as an invader,” Elizabeth Mittendorf, M.D., assistant professor in ht eDepartment of Surgical Oncology at MD Anderson said of the trial that is currently in its second phase. Allan has been receiving the vaccine and it’s boosters since May 2011. “I got my mammogram on April Fools Day of 2010 and I’ll never do that again,” Allan said who ended up having stage tree breast cancer. Allan decided to have a mastectomy and her lymph nodes removed on her right side because she did not want to undergo all of the other treatments. Allan said she wanted to participate in the drug trial because she knew the likelihood of having a reoccurrence. “If it doesn’t help me I hopefully it can help someone else,” Allan said. “I have so many friends that have cancer.”
Sara Stassen, RN, left, at The University of Texas MD Anderson Cancer Center inoculates patient Patricia Anne Allan, 67, of Topeka, Kan., with the AE37 breast cancer vaccine that has show to elicit an immune response in women with varying levels of the human epidermal growth factor receptor2 (HER2) a protein that aids in the progression of certain aggressive types of breast cancer Wednesday, May 16, 2012, in Houston. “The vaccine educates the immune system to recognize HER2 as an invader,” Elizabeth Mittendorf, M.D., assistant professor in ht eDepartment of Surgical Oncology at MD Anderson said of the trial that is currently in its second phase. Allan has been receiving the vaccine and it’s boosters since May 2011. “I got my mammogram on April Fools Day of 2010 and I’ll never do that again,” Allan said who ended up having stage tree breast cancer. Allan decided to have a mastectomy and her lymph nodes removed on her right side because she did not want to undergo all of the other treatments. Allan said she wanted to participate in the drug trial because she knew the likelihood of having a reoccurrence. “If it doesn’t help me I hopefully it can help someone else,” Allan said. “I have so many friends that have cancer.”
It is best known when it occurs in high levels - HER2-positive breast cancers - but it occurs in lower levels in more than half of other breast cancers, plus a number of other cancers, such as prostate, ovarian and gastric cancers. This suggests the drug could provide benefits for patients with these cancers in addition to those with breast cancer.
The vaccine reduced breast cancer recurrence in women who had both high and low levels of HER2.
Breast cancer experts called the study promising, but cautioned that it would premature to make too much of it.
“Vaccines are the exciting new field in cancer, but the data’s just coming in,” said Dr. Julie Nangia, a Baylor College of Medicine professor and the director of the Ben Taub Breast Cancer Risk Assessment and Prevention Clinic. “This study’s promising, but it needs more patients and a longer follow-up to know how well it works.”
The study, to be presented at a cancer conference in June, tested 201 patients. Their average age was 50, they had been treated for intermediate-stage breast cancer, and were disease-free. About half were given the vaccine.
Based on early outcomes, researchers estimated that breast cancer would return in 10 percent of the patients who got the vaccine and 18 percent of those who didn’t - a difference of 43 percent. On average, patients were checked for recurrence 22 months after the vaccine was administered.
Mittendorf said if the vaccine proves effective in later-stage trials, it likely will work in combination with Herceptin, the blockbuster drug now used to treat and prevent recurrence of HER2-positive breast cancers.
But the beauty of the vaccine, she said, is its potential to prevent recurrence of other HER2-affected breast cancer tumors. Some of these still elude targeted therapy, an approach that has advanced cancer care over the last 20 years.
The new breast cancer vaccine - administered to patients “off the shelf” rather than in personalized form - is delivered by injection once a month for six months. After that, a booster shot is given every six months for three years.
The vaccine is one of three undergoing M.D. Anderson trials, including one that stimulates the immune system to fight breast cancer in women with active tumors. The breast cancer prevention vaccine is in an early-stage trial in Greece for patients with prostate cancer.
Mittendorf estimated it will be at least five years before the first breast cancer vaccine is FDA- approved and in widespread use.
###
By Todd Ackerman