Chemotherapy in the Adjuvant Setting
In a similar fashion to tamoxifen, the EBCTCG performed an overview of the worldwide randomized data for the use of adjuvant chemotherapy in early-stage breast cancer (almost 18,000 women randomized in 47 trials). The chemotherapy regimen used in the majority of trials was CMF (cyclophosphamide, methotrexate and 5-fluorouracil [5-FU]).
Chemotherapy significantly reduced the annual risk of recurrence and mortality compared with no chemotherapy, and trials that used a combination of several agents (polychemotherapy) were superior to those of single-agent chemotherapy. The use of prolonged chemotherapy (regimens of typically 1-2 years) did not improve upon the efficacy of shorter regimens (3-6 months).
The benefits of chemotherapy were greatest in women younger than age 50; however, the reductions in the risk of recurrence and death were still significant for women in all age groups (Table 8.2 and Fig. 8.2).
Some have argued that the benefits of chemotherapy in younger patients (typically premenopausal women) are secondary to the actions of chemotherapy on ovarian ablation. Chemotherapy, however, appears to have the greatest impact in the youngest group of patients (age < 40), who are least likely to experience drug-induced amenorrhea. Unfortunately, few women greater than age 70 were randomized in these trials, and therefore insufficient dataexist to comment on the benefits of chemotherapy in this age group.
With that said, there is little reason to believe that women over age 70 do not benefit from chemotherapy; however, the proportional benefits in this age group are not known. Age alone should not be the only determinant for the use of adjuvant chemotherapy in women who are at substantial risk of recurrence.
Chemotherapy has a proportional reduction in the risk of recurrence and death among women with lymph-node positive and node-negative disease similar to that seen with tamoxifen. The absolute benefits of chemotherapy are dependent upon the underlying risk of recurrence, and therefore women with lymph-node positive disease derive the greatest benefit.
Table 8.2. Benefits of adjuvant chemotherapy
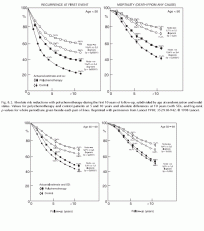 Fig. 8.2. Absolute risk reductions with polychemotherapy during the first 10 years of follow-up, subdivided by age at randomization and nodal status. Values for polychemotherapy and control patients at 5 and 10 years and absolute differences at 10 years (with SDs, and log-rank p-values for whole period) are given beside each pair of lines. Reprinted with permission from Lancet 1998; 352:930-942
Fig. 8.2. Absolute risk reductions with polychemotherapy during the first 10 years of follow-up, subdivided by age at randomization and nodal status. Values for polychemotherapy and control patients at 5 and 10 years and absolute differences at 10 years (with SDs, and log-rank p-values for whole period) are given beside each pair of lines. Reprinted with permission from Lancet 1998; 352:930-942
This concept is best illustrated in Figure 8.3, in which the proportional reductions in mortality are applied to three groups of patients at different risks of recurrence (node-positive, node-negative, and “good prognosis” node-negative with small tumors found on screening mammography).
For women under 50 years of age, absolute improvements in 10 year survival are approximately 11% in the “poor prognosis” lymph-node-positive patients, whereas the “moderate” and “good” prognosis node-negative groups experience smaller improvements in survival of 7% and 2.6%, respectively.
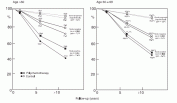 Fig. 8.3. Estimated absolute survival advantages with prolonged polychemotherapy for populations of women with good, intermediate and poor prognosis, subdivided by age (calculated by having the proportional risk reduction unaffected by prognosis). For women over age 50, the absolute improvements in 10 year survival are approximately 3.2% for women at the highest risks of recurrence, and 2.4% and 1.0%, respectively, for women with moderate-and good-prognosis disease. Although the absolute percentages are small, when these improvements in survival are applied to the large number of women who develop breast cancer around the world, the potential number of lives saved is quite large.
Fig. 8.3. Estimated absolute survival advantages with prolonged polychemotherapy for populations of women with good, intermediate and poor prognosis, subdivided by age (calculated by having the proportional risk reduction unaffected by prognosis). For women over age 50, the absolute improvements in 10 year survival are approximately 3.2% for women at the highest risks of recurrence, and 2.4% and 1.0%, respectively, for women with moderate-and good-prognosis disease. Although the absolute percentages are small, when these improvements in survival are applied to the large number of women who develop breast cancer around the world, the potential number of lives saved is quite large.
Some of the trials included in the overview analysis addressed chemotherapy plus tamoxifen versus tamoxifen alone. In women of all ages, the addition of chemotherapy to tamoxifen further reduced the risk of recurrence as compared to treatment with tamoxifen alone. As mentioned previously from the tamoxifen overview, tamoxifen has been shown to add to the benefits of chemotherapy.
Therefore, each treatment appears to contribute independently to improving disease-free and overall survival, and should be considered complementary and not competing adjuvant treatments (Table8.2).
Maura N. Dickler
American Cancer Society Guidelines for the Early Detection of Breast Cancer: Update 2003. CA Cancer J Clin 2003
References
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet 1998; 351:1451-1467.
- Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomized trials. Lancet 1998; 352:930-942.
- Early Breast Cancer Trialists' Collaborative Group. Ovarian ablation in early breast cancer: Overview of the randomized trials. Lancet 1996; 348:1189-1196.
- Fisher B, Costantino JP, Wickerham DL et al. Tamoxifen for the prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst, 1998; 90:1371-1388.
- Gianni Bonadonna, Guest Editor. Breast Cancer. Seminars in Oncology 1996; 23:413-532.