Gene Therapy for Breast Cancer
Review of Clinical Gene Therapy Trials for Breast Cancer and MDR1 Gene Therapy Trial in Cancer Institute Hospital
Gene therapy for advanced breast cancer is anticipated to be a useful therapeutic approach. Strategies in ongoing clinical protocols can be divided into four groups: (1) suppression of oncogenes or transfer of tumor-suppressor genes; (2) enhancement of immunological response; (3) transfer of suicide genes; (4) protection of bone marrow using drug resistance genes. We have started a clinical study of multidrug resistance (MDR1) gene therapy. Advanced breast cancer patients received high dose chemotherapy and autologous peripheral blood stem cell transplantation (PBSCT) with MDR1-transduced hematopoietic cells, and then were treated with docetaxel. Two patients have been treated so far, and in vivo enrichment of MDR1-transduced cells with docetaxel treatment has been seen. Both patients are in complete remission and had no apparent adverse effects from the MDR1 gene transfer.
Breast Cancer 13:8-15, 2006.
Key words: Breast cancer, Gene therapy, MDR1, Adenoviral vector, Retroviral vector
The cure rate of advanced or recurring breast cancer is under 5%, so the usual goal of treatment is prolongation of survival or improvement of quality of life (QOL), not cure1). Endocrine therapy for hormone-receptor-positive patients, chemotherapy, radiation therapy, bisphosphonates for bone diseases, and trastuzumab for HER2-overex-pressed patients, have all been shown to be effective for advanced breast cancer, but none has been shown to increase the cure rate.
Gene therapy for advanced breast cancer is expected to be a useful therapeutic approach.
Strategies in ongoing clinical protocols can be divided into four groups: (1) suppression of oncogenes or transfer of tumor-suppressor genes; (2) enhancement of immunological response; (3) transfer of suicide genes; (4) protection of bone marrow using drug resistance genes (Table 1)2, 3).
There are three major methods for gene transfer: (1) transduction of naked DNA such as lipofection (transient expression); (2) transduction of adenoviral vector or vaccinia virus vector (transient expression); (3) transduction of retroviral vector (stable expression). In this paper, ongoing clinical trials of gene therapy for breast cancer are reviewed, and a clinical trial of multiple drug resistance 1 (MDR1) gene therapy at our institution is described.
Present Status of Clinical Trials of Gene Therapy for Breast Cancer
Suppression of Oncogene Expression or Transfer of Tumor-Suppressor Gene
The carcinogenic process requires an accumulation of multiple gene mutations or abnormalities of gene expression. Common gene abnormalities in breast cancer include p53 gene mutation, ErbB2/ HER2 gene amplification, c-myc gene amplification, and cyclin D1 gene amplification4). Several clinical trials aim to improve those gene abnormalities by local or systemic gene transfer.
A) Transfer of the normal p53 gene: Mutations of the p53 gene are the most frequently found gene abnormalities among various malignancies, including breast cancer5).
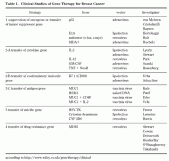 Table 1. Clinical Studies of Gene Therapy for Breast Cancer. Tumor cells with mutated p53 genes show defects of cell-cycle regulation, and transfer of normal p53 genes causes cell-cycle arrest or apoptosis. Clinical studies of p53 gene therapy using adenoviral vectors (Advexin, Introgen et al.) for various tumor types, including breast cancer, are ongoing. Von Mehren and Cristo-fanilli have begun clinical studies of a combination of local injection of p53-adenoviral vector into skin metastatic lesions or locally advanced breast cancer and systemic chemotherapy. Baynes has initiated a clinical study of high dose chemotherapy associated with transplantation of autologous peripheral blood stem cells (PBSC) that have been purged ex vivo by p53-adrnovirus infection. Baynes’s group has shown that p53 gene transfer has no effect on normal PBSC.
Table 1. Clinical Studies of Gene Therapy for Breast Cancer. Tumor cells with mutated p53 genes show defects of cell-cycle regulation, and transfer of normal p53 genes causes cell-cycle arrest or apoptosis. Clinical studies of p53 gene therapy using adenoviral vectors (Advexin, Introgen et al.) for various tumor types, including breast cancer, are ongoing. Von Mehren and Cristo-fanilli have begun clinical studies of a combination of local injection of p53-adenoviral vector into skin metastatic lesions or locally advanced breast cancer and systemic chemotherapy. Baynes has initiated a clinical study of high dose chemotherapy associated with transplantation of autologous peripheral blood stem cells (PBSC) that have been purged ex vivo by p53-adrnovirus infection. Baynes’s group has shown that p53 gene transfer has no effect on normal PBSC.
B) Suppression of the ErbB2/HER2 gene: The ErbB2/HER2 gene encodes an 185 kD protein and is a member of the epidermal growth-factor receptor family. This gene is amplified in 20-30% of breast cancer patients, and correlates with a poor prognosis and resistance to hormone therapy4).
Monoclonal humanized murine antibody to ErbB2/HER2 protein (trastuzumab/Herceptin™) is effective in advanced, ErbB2/HER2-overexpressing breast cancer patients6). The adenovirus type 2 or type 5 E1A gene inhibits expression of the ErbB2/HER2 gene, and E1A gene transfer into ErbB2/ HER2-overexpressed tumors causes tumor reduction and enhances sensitivity to chemotherapy in vitro and in vivo7). At MD Anderson Cancer Center, patients with breast cancer or ovarian cancer overexpressing ErbB2/HER2 were treated with gene therapy using a local injection of E1A gene-liposome into skin lesions or pleural/peritoneal effusion8). There was no serious adverse effect other than fever or pain at the injection sites. In six cases in which tumor cells in body fluids could be analyzed, reduction of ErbB2/HER2 expression and a decrease in tumor cells were shown.
E1A gene transfer also reduced tumor growth of non-HER2-overexpressing cells, and E1A gene transfer to tumor tissues of breast cancer or head and neck cancer by lipofection showed minor response in HER2-negative tumors9).
C) Suppression of c-myc and c-fos gene: Arteaga and Holt made a retroviral vector which over-expresses antisense mRNA to c-myc and c-fos genes under the control of mammary tumor virus (MMTV) promoter. Transfer of this vector into a breast cancer cell line suppressed tumor formation in animal models10). They have started a clinical trial of gene therapy for malignant effusion or meningitis in breast cancer patients who have failed standard therapy. Effusions will be drained and replaced with a solution of the vector, then periodically drained to follow the disease and assess gene transfer11).
D) Transfer of melanoma differentiation associated protein 7 (MDA-7): MDA-7 is a novel tumor suppressor gene, and its transfer into tumor cells causes growth suppression and apoptosis. However, MDA-7 gene transfer into normal cell lines does not12). A clinical trial of gene therapy that injects MDA-7- adenoviral vector (Ad-mda7, ISGN 241) into tumor cells has started (Buchholz).
There was no serious adverse effect in a phase I study, and a combination phase I/II study with irradiation has begun.