Tamoxifen Use and Endometrial Abnormalities
In the past 25 years, several million women have been treated with tamoxifen. Currently, the recommended duration of adjuvant tamoxifen treatment in women with breast cancer is 5 years.
Tamoxifen is an antiestrogen, but it also acts as a partial estrogen agonist on the endometrium. Administration of unopposed estrogen can lead to endometrial proliferation and occasionally to carcinoma. Tamoxifen use has been associated with a variety of histopathologic changes in the endometrium, including increased endometrial thickness, increased uterine volume, proliferative changes, simple and complex atypical endometrial hyperplasia, endometrial polyps, and, rarely, endometrial carcinoma.
In the National Surgical Adjuvant Breast and Bowel Project P-1 trial, which included patients at increased risk for the development of breast cancer and which examined the value of tamoxifen in preventing second breast cancers, patients were randomly assigned to receive either tamoxifen 20 mg daily or placebo. The cumulative rate of endometrial cancer was 13.0 cases per 1,000 women in the tamoxifen group and 5.4 cases per 1,000 women in the placebo control group.
The increased risk in tamoxifen-treated women appeared at 1 year of tamoxifen treatment and increased progressively with treatment durations beyond 5 years. The relative risk of developing endometrial cancer after tamoxifen treatment increases with higher cumulative doses of tamoxifen and longer duration of exposure.
Recently, results of the Study of Tamoxifen and Raloxifene showed that another selective estrogen receptor modulator, raloxifene, is as effective as tamoxifen in reducing the incidence of breast cancer in postmenopausal women who are at increased risk for the disease and is associated with a lower risk of endometrial cancer (Land et al., 2006). Women who were randomly assigned to take raloxifene had 36% fewer uterine cancers than the women assigned to take tamoxifen. It is postulated that raloxifene may become more widely used than tamoxifen for breast cancer prevention in the near future.
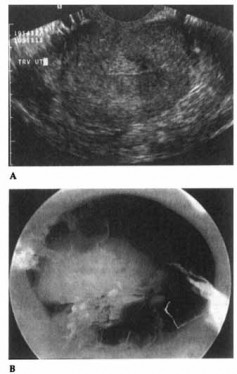 Figure 15-2. Endometrial polyp as seen on vaginal sonography (A) and hysteroscopy (B).
Figure 15-2. Endometrial polyp as seen on vaginal sonography (A) and hysteroscopy (B).
In women treated for breast cancer, the benefits of tamoxifen in terms of reducing the risk of breast cancer recurrence far outweigh the small risk of endometrial cancer. However, because of the risk of endometrial cancer, women taking tamoxifen must have frequent and thorough examinations.
Table 15-4 shows the American College of Obstetrics and Gynecology guidelines for care of patients undergoing treatment with tamoxifen (ACOG, 2006).
Sonography has proven to be useful for evaluating the endometrium in patients taking tamoxifen who have abnormal vaginal bleeding. Typical sonographic appearances of the endometrium in patients taking tamoxifen include thick, homogeneous hyperechogenic tissue with small cystic spaces; heterogeneous tissue with small cystic spaces; and solid heterogeneous tissue and polyps.
Table 15-4. American College of Obstetrics and Gynecology Recommendations for the Care of Women Taking Tamoxifen.
- Postmenopausal women taking tamoxifen should be monitored closely for symptoms of endometrial hyperplasia or cancer.
- Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.
- Women taking tamoxifen should be informed about the risks of endometrial proliferation, endometrial hyperplasia, endometrial cancer, and uterine sarcomas. Women should be encouraged to promptly report any abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Any abnormal vaginal bleeding, bloody vaginal discharge, staining, or spotting should be investigated.
- Emerging evidence suggests the presence of high- and low-risk groups for development of atypical hyperplasias with tamoxifen treatment in postmenopausal women based on the presence or absence of benign endometrial polyps before therapy. Thus there may be a role for pretreatment screening of postmenopausal women with transvaginal ultrasonography, and sonohysterography when needed, or office hysteroscopy before initiation of tamoxifen therapy.
- Unless the patient has been identified to be at high risk for endometrial cancer, routine endometrial surveillance has not been effective in increasing the early detection of endometrial cancer in women using tamoxifen. Such surveillance may lead to more invasive and costly diagnostic procedures and, therefore, is not recommended.
- Tamoxifen use should be limited to 5 years’ duration because a benefit beyond this time has not been documented.
- If atypical endometrial hyperplasia develops, appropriate gynecologic management should be instituted, and the use of tamoxifen should be reassessed. If tamoxifen therapy must be continued, hysterectomy should be considered in women with atypical endometrial hyperplasia. Tamoxifen use may be reinstituted following hysterectomy for endometrial carcinoma in consultation with the physician responsible for the women’s breast care.
When 5 mm is used as the upper limit of normal endometrial thickness, the sensitivity of transvaginal sonography for the detection of endometrial abnormalities is 91-100%. An endometrial thickness of greater than 10 mm is almost always associated with some type of endometrial abnormality, such as hyperplasia or polyps.
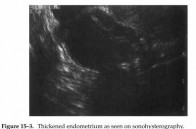 Figure 15-3. Thickened endometrium as seen on sonohysterography.
Figure 15-3. Thickened endometrium as seen on sonohysterography.
Figures 15-2A and 15-2B show an endometrial polyp as seen on vaginal sonography and hysteroscopy, respectively.
Some investigators have utilized transvaginal pulse Doppler color flow imaging and have concluded that it does not contribute to the assessment of asymptomatic postmenopausal breast cancer patients treated with tamoxifen. Another technique often utilized is sonohysterography. This involves filling the endometrial cavity with saline under sonographic visualization, thus distending the cavity and allowing more effective analysis. Sonohysterography is particularly useful for delineating polyps and space-occupying lesions, such as submucosal myomas, and for identifying a thickened endometrium (Figure 15-3).
In asymptomatic women taking tamoxifen, screening for endometrial cancer with routine transvaginal sonography, endometrial biopsy, or both has not been shown to be effective. The likelihood of detecting endometrial pathology in asymptomatic patients is very low, usually between 0.6% and 4%, and most abnormal findings do not require specific treatment. Any patient who has vaginal bleeding or a bloody vaginal discharge while taking tamoxifen should undergo immediate endometrial biopsy regardless of the thickness of the endometrial lining on sonography.
Elizabeth R. Keeler, Pedro T. Ramirez, and Ralph S. Freedman
Committee on Gynecological Practice, the American College of Obstetricians and Gynecologists. Obstet Gynecol 2007