Developing Technologies for the Early Detection of Breast Cancer
Breast cancer is the most common non-skin-related malignancy and the second leading cause of cancer death among women in the United States1 . Each year, more than 180,000 new cases of invasive breast cancer are diagnosed and more than 40,000 women die from the disease. Until research uncovers a way to prevent breast cancer or to cure all women regardless of when their tumor is found, early detection will be looked upon as the best hope for reducing the heavy toll of this disease. The early detection of cervical cancer by screening with the Papanicolaou smear (the Pap smear) dramatically reduced mortality from that cancer, and the rationale for the early detection of breast cancer is similar.
Fifty years ago, there was no established method for the detection of breast cancer at an early stage or for screening of the general population, but advances in technology, policy recommendations by various organizations, and legal mandates have thoroughly changed that situation (Figure 1-1). Although the use of X-ray imaging for the detection of breast cancer was first suggested in the early 1900s, mammography did not begin to emerge as an accepted technology until the 1960s, after a number of technical innovations that produced higher-quality images that were more reproducible and easier to interpret were introduced. Subsequently, some physicians began ordering mammograms to help with the diagnosis of complicated cases, and the technology was also tested as a screening tool (reviewed by Lerner, 2001).
X-ray film mammography and physical examination of the breast are now the mainstays for early detection of breast cancer. Screening for early cancer detection has been credited for part of the recent reduction in breast cancer mortality, which had been stagnant for 40 years (Blanks et al., 2000; Hakama et al., 1997; Mettlin, 1999; Peto et al., 2000). (Adjuvant therapy is also credited with reducing breast cancer mortality). New or improved technologies are also rapidly emerging and providing new hope of early detection.
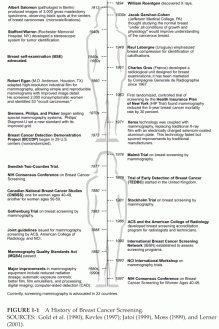 FIGURE 1-1 A History of Breast Cancer Screening.
FIGURE 1-1 A History of Breast Cancer Screening.
SOURCES: Gold et al. (1990), Kevles (1997); Jatoi (1999), Moss (1999), and Lerner (2001).
Over the past decade, the investment in breast cancer research, including early detection, has increased substantially. Research has intensified with federal funding, and private firms have turned more attention to breast cancer detection. Programs within the U.S. Department of Health and Human Services and the U.S. Department of Defense support large numbers of investigators working on breast cancer, and recently, the National Aeronautics and Space Administration and several intelligence services have agreed to apply their imaging expertise to mammography (Table 1-1). Biotechnology and device companies have proliferated, with many developing technologies that might improve the ability to detect breast cancer early, and established firms have also turned their attention to breast cancer, in part as the result of findings derived from the rising federal research investment.
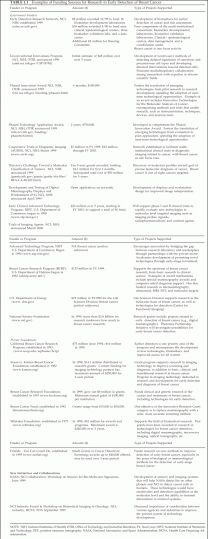 TABLE 1.1 Examples of Funding Sources for Research in Early Detection of Breast Cancer
TABLE 1.1 Examples of Funding Sources for Research in Early Detection of Breast Cancer
Advances in imaging include reducing the dose of X rays needed, enhancement of digital images, computer-assisted analysis of images, and use of alternatives to X rays such as ultrasound, magnetic resonance imaging (MRI), and optical imaging. Techniques for high-resolution imaging and image processing, many of which were developed for other applications such as space science, are now being applied to breast imaging, with hopes of improved accuracy, speed, ease of use, and perhaps lower cost. Advances in genetics and increased knowledge of the basic biology and etiology of breast cancer may also lead to novel, biologically based early detection and diagnostic methods. Use of molecular markers may increase the accuracy of diagnostic techniques and offer new opportunities for the characterization of early disease as well as for the refinement and improvement of treatments.
However, early detection depends on more than just the development of technologies and the advance of new science. Technological advances must be thoroughly evaluated before they can become widely used by women. This evaluation takes place in many stages, including Food and Drug Administration (FDA) approval (when it is a device), adoption by health plans and providers, approval of payment for screening and detection, acceptance by women, and marketing by private firms.