Health Centers > Diabetes Center > Diabetes Mellitus & Hypoglycemia
Diabetes Mellitus & Hypoglycemia
Essentials of diagnosis
Type 1 Diabetes:
- Polyuria, polydipsia, and weight loss associated with random plasma glucose ≥ 200 mg/dL.
- Plasma glucose of 126 mg/dL or higher after an overnight fast, documented on more than one occasion.
- Ketonemia, ketonuria, or both.
- Islet autoantibodies are frequently present.
Type 2 Diabetes:
- Most patients are over 40 years of age and obese.
- Polyuria and polydipsia. Ketonuria and weight loss generally are uncommon at time of diagnosis. Candidal vaginitis in women may be an initial manifestation. Many patients have few or no symptoms.
- Plasma glucose of 126 mg/dL or higher after an overnight fast on more than one occasion. After 75 g oral glucose, diagnostic values are 200 mg/dL or more 2 hours after the oral glucose.
- Hypertension, dyslipidemia, and atherosclerosis are often associated.
Diabetes Mellitus
Essentials of diagnosis
Epidemiologic Considerations
Classification & Pathogenesis
L Type 1 Diabetes Mellitus
L Type 2 Diabetes Mellitus
L Other specific types of Diabetes Mellitus
L Insulin Resistance Syndrome
Clinical Findings
L Symptoms and sings
L Laboratory findings
L Differential Diagnosis
Clinical Trials in Diabetes
LType 1 Diabetes
L Type 2 Diabetes
Treatment Regimens
L Diet
L Drugs for treating hyperglycemia
L Insulin
L Pancreas transplantation
L General Considerations in Treatment
Steps in the Management of the Diabetic Patient
L Diagnostic Examination
L Patient Education (Self-Management training)
Diabetes Mellitus Treatment
L Type 2 diabetes
L Type 1 diabetes
L Acceptable Levels of Glycemic Control
Complications of Insulin Therapy
Epidemiologic Considerations
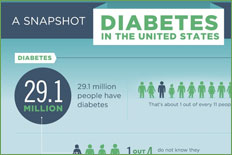
In 2002, an estimated 18.2 million people in the United States had diabetes mellitus, of which approximately 1 million have type 1 diabetes and the rest mostly have type 2 diabetes. A third group that was designated as "other specific types" by the American Diabetes Association (ADA) number only in the thousands. Among these are the rare monogenic defects of either B cell function or of insulin action, primary diseases of the exocrine pancreas, endocrinopathies, and drug-induced diabetes. Updated information about the prevalence of diabetes in the United States is available from the Centers for Disease Control and Prevention (http://www.cdc.gov/diabetes/pubs/estimates.htm).
Classification & Pathogenesis
Diabetes mellitus is a syndrome with disordered metabolism and inappropriate hyperglycemia due to either a deficiency of insulin secretion or to a combination of insulin resistance and inadequate insulin secretion to compensate. Type 1 diabetes is due to pancreatic islet B cell destruction predominantly by an autoimmune process, and these patients are prone to ketoacidosis. Type 2 diabetes is the more prevalent form and results from insulin resistance with a defect in compensatory insulin secretion.
The Hypoglycemic States
Spontaneous hypoglycemia in adults is of two principal types: fasting and postprandial. Symptoms begin ...
A. Type 1 Diabetes Mellitus
This form of diabetes is immune-mediated in over 90% of cases and idiopathic in less than 10%. The rate of pancreatic B cell destruction is quite variable, being rapid in some individuals and slow in others. Type 1 diabetes is usually associated with ketosis in its untreated state. It occurs at any age but most commonly arises in children and young adults with a peak incidence before school age and again at around puberty. It is a catabolic disorder in which circulating insulin is virtually absent, plasma glucagon is elevated, and the pancreatic B cells fail to respond to all insulinogenic stimuli. Exogenous insulin is therefore required to reverse the catabolic state, prevent ketosis, reduce the hyperglucagonemia, and reduce blood glucose.
The highest incidence of immune-mediated type 1 diabetes is in Scandinavia and northern Europe, where the yearly incidence per 100,000 youngsters 14 years of age or less is as high as 37 in Finland, 27 in Sweden, 22 in Norway, and 19 in the United Kingdom. The incidence of type 1 diabetes generally decreases across the rest of Europe to 10 in Greece and 8 in France. Surprisingly, the island of Sardinia has as high an incidence as Finland (37) even though in the rest of Italy, including the island of Sicily, it is only 10 per 100,000 per year. The United States averages 15 per 100,000, with higher incidences in states more densely populated with persons of Scandinavian descent such as Minnesota. The lowest incidence of type 1 diabetes worldwide was found to be less than 1 per 100,000 per year in China and parts of South America. The global incidence of type 1 diabetes is increasing (approximately 3% each year).
Certain HLAs are strongly associated with the development of type 1 diabetes. About 95% of patients with type 1 diabetes possess either HLA-DR3 or HLA-DR4, compared with 45-50% of white controls. HLA-DQ genes are even more specific markers of type 1 susceptibility, since a particular variety (HLA-DQB1*0302) is found in the DR4 patients with type 1, while a "protective" gene (HLA-DQB1*0602) is often present in the DR4 controls. In addition, most patients with type 1 diabetes at diagnosis have circulating antibodies to islets (islet cell antibodies, ICA), insulin (IAA), glutamic acid decarboxylase (GAD65), and to tyrosine phosphatases (IA-2 and IA2-β). These antibodies facilitate screening of siblings of affected children as well as adults with atypical features of type 2 for an autoimmune cause of their diabetes. The antibody levels decline with increasing duration of the disease. Also, once patients are treated with insulin, low levels of anti-insulin antibodies develop.
Family members of diabetic probands are at increased lifetime risk for developing type 1 diabetes. The offspring of a mother with type 1 diabetes has a risk of 3%, whereas the risk is 6% if the father is affected. The risk in siblings is related to the number of HLA haplotypes that the sibling shares with the diabetic proband. If one haplotype is shared, the risk is 6% and if two haplotypes are shared, the risk increases to 12-25%. The highest risk is for identical twins, where the concordance rate is 25-50%.
Certain unrecognized patients with a milder expression of type 1 diabetes initially retain enough B cell function to avoid ketosis but as their B cell mass diminishes later in life, dependence on insulin therapy develops. Islet cell antibody surveys among northern Europeans indicate that up to 15% of "type 2" patients may actually have this mild form of type 1 diabetes (latent autoimmune diabetes of adulthood; LADA).
1. Immune-mediated type 1 diabetes mellitus - Immune-mediated type 1 diabetes is believed to result from an infectious or toxic insult to persons whose immune system is genetically predisposed to develop a vigorous autoimmune response either against altered pancreatic B cell antigens or against molecules of the B cell resembling the viral protein (molecular mimicry). Extrinsic factors that affect B cell function include damage caused by viruses such as mumps or coxsackie B4 virus, by toxic chemical agents, or by destructive cytotoxins and antibodies released from sensitized immunocytes. Specific HLA immune response genes are believed to predispose patients to a destructive autoimmune response against their own islet cells (autoaggression), which is mediated primarily by cytotoxic T cells. Amelioration of hyperglycemia in patients given an immunosuppressive agent (eg, cyclosporine) shortly after onset of type 1 diabetes lends further support to the pathogenetic role of autoimmunity.
2. Idiopathic type 1 diabetes mellitus - Fewer than 10% of subjects have no evidence of pancreatic B cell autoimmunity to explain their insulinopenia and ketoacidosis. This subgroup has been classified as "idiopathic type 1 diabetes" and designated as "type 1B." Although only a minority of patients with type 1 diabetes fall into this group, most of these are of Asian or African origin. It was recently reported that about 4% of the West Africans with ketosis-prone diabetes are homozygous for a mutation in PAX-4 (Arg133Trp) - a gene that is essential for the development of pancreatic islets.
B. Type 2 Diabetes Mellitus
This represents a heterogeneous group of conditions that used to occur predominantly in adults, but it is now more frequently encountered in children and adolescents. More than 90% of all diabetic persons in the United States are included under this classification. Circulating endogenous insulin is sufficient to prevent ketoacidosis but is inadequate to prevent hyperglycemia in the face of increased needs owing to tissue insensitivity. In most cases of this type of diabetes, the cause is unknown.
Tissue insensitivity to insulin has been noted in most type 2 patients irrespective of weight and has been attributed to several interrelated factors. These include a putative (and as yet undefined) genetic factor, which is aggravated in time by additional enhancers of insulin resistance such as aging, a sedentary lifestyle, and abdominal-visceral obesity. In addition, there is an accompanying deficiency in the response of pancreatic B cells to glucose. Both the tissue resistance to insulin and the impaired B cell response to glucose appear to be further aggravated by increased hyperglycemia (glucose toxicity), and both defects are ameliorated by treatment that reduces the hyperglycemia toward normal. Most epidemiologic data indicate strong genetic influences, since in monozygotic twins over 40 years of age, concordance develops in over 70% of cases within a year whenever type 2 diabetes develops in one twin. Attempts to identify genetic markers for type 2 have as yet been unsuccessful, although linkage to a gene on chromosome 2 encoding a cysteine protease, calpain-10, has been reported in a Mexican-American population. However, its association with other ethnic populations and any role it plays in the pathogenesis of type 2 diabetes remain to be clarified.
The degree and prevalence of obesity varies among different racial groups with type 2 diabetes. While obesity is apparent in no more than 30% of Chinese and Japanese patients with type 2, it is found in 60-70% of North Americans, Europeans, or Africans with type 2 and approaches 100% of patients with type 2 among Pima Indians or Pacific Islanders from Nauru or Samoa.
Patients with this most common form of diabetes have an insensitivity to endogenous insulin. When an associated defect of insulin production prevents adequate compensation for this insulin resistance, nonketotic mild diabetes occurs. Hyperplasia of pancreatic B cells is often present and probably accounts for the fasting hyperinsulinism and exaggerated insulin and proinsulin responses to glucose and other stimuli seen early in the disease. After several years' duration of diabetes, chronic deposition of amyloid in the islets may combine with inherited genetic defects to progressively impair B cell function.
The mechanisms underlying the insulin resistance of type 2 diabetes are poorly understood. Obesity is generally associated with abdominal distribution of fat, producing an abnormally high waist-to-hip ratio. This "visceral" obesity, due to accumulation of fat in the omental and mesenteric regions, correlates with insulin resistance; subcutaneous abdominal fat seems to have less of an association with insulin insensitivity. Exercise may affect the deposition of visceral fat as suggested by CT scans of Japanese wrestlers, whose extreme obesity is predominantly subcutaneous. Their daily vigorous exercise program prevents accumulation of visceral fat, and they have normal serum lipids and euglycemia despite daily intakes of 5000-7000 kcal and development of massive subcutaneous obesity. Several adipokines, secreted by fat cells, can affect insulin action in obesity. Two of these, leptin and adiponectin, seem to increase sensitivity to insulin, presumably by increasing hepatic responsiveness. Two others - tumor necrosis factor-α, which inactivates insulin receptors, and the newly discovered peptide resistin - interfere with insulin action on glucose metabolism and have been reported to be elevated in obese animal models. Mutations or abnormal levels of these adipokines may contribute to the development of insulin resistance in human obesity.
Hyperglycemia per se can impair insulin action by causing accumulation of hexosamines in muscle and fat tissue and inhibiting glucose transport (acquired glucose toxicity). Correction of hyperglycemia reverses this acquired insulin resistance.
C. Other specific types of Diabetes Mellitus
1. Maturity-onset diabetes of the young (MODY) - This subgroup is a relatively rare monogenic disorder characterized by non-insulin-dependent diabetes with autosomal dominant inheritance and an age at onset of 25 years or younger. Patients are nonobese, and their hyperglycemia is due to impaired glucose-induced secretion of insulin. Six types of MODY have been described. Except for MODY 2, in which a glucokinase gene is defective, all other types involve mutations of a nuclear transcription factor that regulates islet gene expression.
MODY 2 is quite mild, associated with only slight fasting hyperglycemia and few if any microvascular diabetic complications. It generally responds well to hygienic measures or low doses of oral hypoglycemic agents. MODY 3 - the most common form - accounts for two-thirds of all MODY cases. The clinical course is similar to that of idiopathic type 2 diabetes in terms of microangiopathy and failure to respond to oral agents with time.
2. Diabetes due to mutant insulins - This is a very rare subtype of nonobese type 2 diabetes, with no more than ten families having been described. Since affected individuals were heterozygous and possessed one normal insulin gene, diabetes was mild, did not appear until middle age, and showed autosomal dominant genetic transmission. There is generally no evidence of clinical insulin resistance, and these patients respond well to standard therapy.
3. Diabetes due to mutant insulin receptors - Defects in one of their insulin receptor genes have been found in more than 40 people with diabetes, and most have extreme insulin resistance associated with acanthosis nigricans. In very rare instances when both insulin receptor genes are abnormal, newborns present with a leprechaun-like phenotype and seldom live through infancy.
4. Diabetes mellitus associated with a mutation of mitochondrial DNA - Since sperm do not contain mitochondria, only the mother transmits mitochondrial genes to her offspring. Diabetes due to a mutation of mitochondrial DNA that impairs the transfer of leucine or lysine into mitochondrial proteins has been described. Most patients have a mild form of diabetes that responds to oral hypoglycemic agents; some have a nonimmune form of type 1 diabetes. Two-thirds of patients with this subtype of diabetes have a hearing loss, and a smaller proportion (15%) had a syndrome of myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS).
5. Wolfram's syndrome - Wolfram's syndrome is an autosomal recessive neurodegenerative disorder first evident in childhood. It consists of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, hence the acronym DIDMOAD. It is due to mutations in a gene named WFS1, which encodes a 100.3 KDa transmembrane protein localized in the endoplasmic reticulum. The function of the protein is not known. The diabetes mellitus, which is nonimmune and not linked to specific HLA antigens, usually presents in the first decade together with the optic atrophy. Cranial diabetes insipidus and sensorineural deafness develop during the second decade in 60-75% of patients. Ureterohydronephrosis, neurogenic bladder, cerebellar ataxia, peripheral neuropathy, and psychiatric illness develop later in many patients.
Insulin Resistance Syndrome (Syndrome X; Metabolic Syndrome)
Twenty-five percent of the general nonobese nondiabetic population has insulin resistance of a magnitude similar to that seen in type 2 diabetes. These insulin-resistant nondiabetic individuals are at much higher risk for developing type 2 diabetes than insulin-sensitive persons. In addition to diabetes, these individuals have increased risk for elevated plasma triglycerides, lower high-density lipoproteins (HDLs), and higher blood pressure - a cluster of abnormalities termed syndrome X. These associations have now been expanded to include small, dense, low-density lipoprotein (LDL), hyperuricemia, abdominal obesity, prothrombotic state with increased levels of plasminogen activator inhibitor type 1 (PAI-1), and proinflammatory state. These clusters of abnormalities significantly increase the risk of atherosclerotic disease.
It has been postulated that hyperinsulinemia and insulin resistance play a direct role in these metabolic abnormalities, but supportive evidence is inconclusive. Although hyperinsulinism and hypertension often coexist in whites, that is not the case in blacks or Pima Indians. Moreover, patients with hyperinsulinism due to insulinoma are not hypertensive, and there is no fall in blood pressure after surgical removal of the insulinoma restores normal insulin levels. The main value of grouping these disorders as a syndrome, however, is to remind clinicians that the therapeutic goals are not only to correct hyperglycemia but also to manage the elevated blood pressure and dyslipidemia that result in increased cerebrovascular and cardiac morbidity and mortality in these patients. Clinicians aware of this syndrome are more cautious in prescribing therapies that correct hypertension but may raise lipids (diuretics, β-blockers) or that correct hyperlipidemia but increase insulin resistance, with aggravation of diabetes (niacin). Finally, the use of long-acting insulins and sulfonylureas that promote sustained hyperinsulinism may have to be moderated, with insulin-sparing drugs such as metformin or a thiazolidinedione being preferable, if the hypothesis behind the insulin resistance syndrome is ever substantiated.