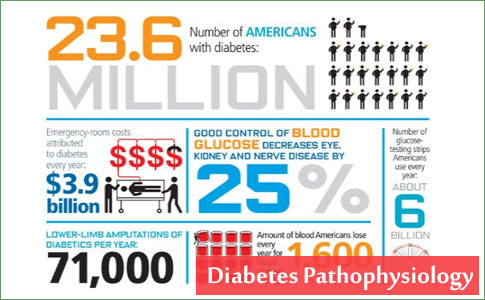
Health Centers > Diabetes Center > Diabetes Pathophysiology
Diabetes Pathophysiology
Introduction
An understanding of the pathophysiology of diabetes rests upon knowledge of the basics of carbohydrate metabolism and insulin action. Following the consumption of food, carbohydrates are broken down into glucose molecules in the gut. Glucose is absorbed into the bloodstream elevating blood glucose levels. This rise in glycemia stimulates the secretion of insulin from the beta cells of the pancreas. Insulin is needed by most cells to allow glucose entry. Insulin binds to specific cellular receptors and facilitates entry of glucose into the cell, which uses the glucose for energy. The increased insulin secretion from the pancreas and the subsequent cellular utilization of glucose results in lowered of blood glucose levels. Lower glucose levels then result in decreased insulin secretion.
Diabetes Mellitus and Oral Diseases
Diabetes Introduction
Diabetes Epidemiology and classification
Diabetes Pathophysiology
Clinical Presentation, Laboratory findings, and Diagnosis
Diabetes Complications
Diabetes Management
L Introduction
L Oral Agents
L Insulin
Oral Diseases and Diabetes
Periodontal Health and Diabetes
Dental Management of the Diabetic Patient
Conclusion
References
If insulin production and secretion are altered by disease, blood glucose dynamics will also change. If insulin production is decreased, glucose entry into cells will be inhibited, resulting in hyperglycemia. The same effect will be seen if insulin is secreted from the pancreas but is not used properly by target cells. If insulin secretion is increased, blood glucose levels may become very low (hypoglycemia) as large amounts of glucose enter tissue cells and little remains in the bloodstream.
Following meals, the amount of glucose available from carbohydrate breakdown often exceeds the cellular need for glucose. Excess glucose is stored in the liver in the form of glycogen, which serves as a ready reservoir for future use. When energy is required, glycogen stores in the liver are converted into glucose via glycogenolysis, elevating blood glucose levels and providing the needed cellular energy source. The liver also produces glucose from fat (fatty acids) and proteins (amino acids) through the process of gluconeogenesis. Glycogenolysis and gluconeogenesis both serve to increase blood glucose levels. Thus, glycemia is controlled by a complex interaction between the gastrointestinal tract, the pancreas, and the liver.
Multiple hormones may affect glycemia. Insulin is the only hormone that lowers blood glucose levels. The counter-regulatory hormones such as glucagon, catecholamines, growth hormone, thyroid hormone, and glucocorticoids all act to increase blood glucose levels, in addition to their other effects.
Type 1 Diabetes
The underlying pathophysiologic defect in type 1 diabetes is an autoimmune destruction of pancreatic beta cells. Following this destruction, the individual has an absolute insulin deficiency and no longer produces insulin. Autoimmune beta cell destruction is thought to be triggered by an environmental event, such as a viral infection. Genetically determined susceptibility factors increase the risk of such autoimmune phenomena.
The onset of type 1 diabetes is usually abrupt. It generally occurs before the age of 30 years, but may be diagnosed at any age. Most type 1 diabetic individuals are of normal weight or are thin in stature. Since the pancreas no longer produces insulin, a type 1 diabetes patient is absolutely dependent on exogenously administered insulin for survival. People with type 1 diabetes are highly susceptible to diabetic ketoacidosis. Because the pancreas produces no insulin, glucose cannot enter cells and remains in the bloodstream. To meet cellular energy needs, fat is broken down through lipolysis, releasing glycerol and free fatty acids. Glycerol is converted to glucose for cellular use. Fatty acids are converted to ketones, resulting in increased ketone levels in body fluids and decreased hydrogen ion concentration (pH). Ketones are excreted in the urine, accompanied by large amounts of water. The accumulation of ketones in body fluids, decreased pH, electrolyte loss and dehydration from excessive urination, and alterations in the bicarbonate buffer system result in diabetic ketoacidosis (DKA). Untreated DKA can result in coma or death.
The Hypoglycemic States
Spontaneous hypoglycemia in adults is of two principal types: fasting and postprandial. Symptoms begin ...
Many patients with type 1 diabetes are initially diagnosed with the disease following a hospital admission for DKA. In a known diabetic patient, periods of stress or infection may precipitate DKA. More often, however, DKA results from poor daily glycemic control. Patients who remain severely hyperglycemic for several days or longer due to inadequate insulin administration or excessive glucose intake are prone to developing DKA.
Type 2 Diabetes
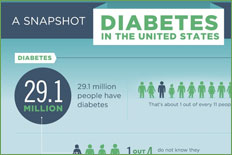
About 90% of diabetic Americans have type 2 diabetes. The prevalence of type 2 diabetes is higher in African Americans, Native Americans, Hispanics, and Pacific Islanders than it is in Caucasians. Most type 2 diabetes patients are overweight, and most are diagnosed as adults. The genetic influence in type 2 diabetes is greater than that seen with type 1. While concordance rates between monozygous twins for type 1 diabetes are about 30 to 50%, the rate is approximately 90% for type 2 diabetes. Although the genetic predisposition to type 2 diabetes is strong, no single genetic defect has been found. In addition to genetic influences, acquired risk factors for type 2 diabetes include obesity, advancing age, and an inactive lifestyle.
The underlying pathophysiologic defect in type 2 diabetes does not involve autoimmune beta-cell destruction. Rather, type 2 diabetes is characterized by the following three disorders: (1) peripheral resistance to insulin, especially in muscle cells; (2) increased production of glucose by the liver; and, (3) altered pancreatic insulin secretion. Increased tissue resistance to insulin generally occurs first and is eventually followed by impaired insulin secretion. The pancreas produces insulin, yet insulin resistance prevents its proper use at the cellular level. Glucose cannot enter target cells and accumulates in the bloodstream, resulting in hyperglycemia. The high blood glucose levels often stimulate an increase in insulin production by the pancreas; thus, type 2 diabetic individuals often have excessive insulin production (hyperinsulinemia). Over the years, pancreatic insulin production usually decreases to below normal levels. In addition to hyperglycemia, type 2 diabetic patients often have a group of disorders that has been called "insulin resistance syndrome" or syndrome X.
Obesity contributes greatly to insulin resistance, even in the absence of diabetes. In fact, weight loss is a cornerstone of therapy for obese type 2 diabetic patients. Insulin resistance generally decreases with weight loss. Obesity also may explain the dramatic increase in the incidence of type 2 diabetes among young individuals in the United States in the past 10 to 20 years. Once considered a disease of adults, type 2 diabetes has increased among America's youth in direct correlation with the increase in the average weight of children and young adults during that time period.
Type 2 diabetes usually has a slow onset and may remain undiagnosed for years. Approximately half of those who have type 2 diabetes are unaware of their disease. Unfortunately, the insidious nature of the disease allows prolonged periods of hyperglycemia to begin exerting negative effects on major organ systems. By the time many type 2 diabetic patients are diagnosed, diabetic complications have already begun. Type 2 diabetic patients do not require exogenous insulin for survival since they still produce insulin. However, insulin injection is often an integral part of medical management for type 2 diabetes. Unlike type 1 diabetic patients, individuals with type 2 diabetes are generally resistant to DKA because their pancreatic insulin production is often sufficient to prevent ketone formation. Severe physiologic stress may induce DKA in those with type 2 diabetes. Long periods of severe hyperglycemia may result in hyperosmolar nonketotic acidosis. Hyperglycemia results in the urinary excretion of large amounts of glucose, with attendant water loss. If fluids are not replaced, the dehydration can result in electrolyte imbalance and acidosis.
Gestational Diabetes
Gestational diabetes occurs in approximately 4% of pregnancies in the United States. It usually develops during the third trimester and significantly increases perinatal morbidity and mortality.11 The proper diagnosis and management of gestational diabetes improves pregnancy outcomes. As with type 2 diabetes, the pathophysiology of gestational diabetes is associated with increased insulin resistance. Most patients with gestational diabetes return to a normoglycemic state after parturition; however, about 30 to 50% of women with a history of gestational diabetes will develop type 2 diabetes within 10 years.
Impaired Glucose Tolerance and Impaired Fasting Glucose
The conditions known as impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) represent metabolic states lying between diabetes and normoglycemia. People with IFG have increased fasting blood glucose levels but usually have normal levels following food consumption. Those with IGT are normoglycemic most of the time but can become hyperglycemic after large glucose loads. IGT and IFG are not considered to be clinical entities; rather, they are risk factors for future diabetes. The pathophysiology of IFG and IGT is related primarily to increased insulin resistance whereas endogenous insulin secretion is normal in most patients. Approximately 30 to 40% of individuals with IGT or IFG will develop type 2 diabetes within 10 years after onset.