Pathophysiology of adult fecal incontinence
Abstract
Fecal incontinence occurs when the normal anatomy or physiology that maintains the structure and function of the anorectal unit is disrupted. Incontinence usually results from the interplay of multiple pathogenic mechanisms and is rarely attributable to a single factor. The internal anal sphincter (IAS) provides most of the resting anal pressure and is reinforced during voluntary squeeze by the external anal sphincter (EAS), the anal mucosal folds, and the anal endovascular cushions.
Disruption or weakness of the EAS can cause urge-related or diarrhea-associated fecal incontinence. Damage to the endovascular cushions may produce a poor anal “seal” and an impaired anorectal sampling reflex. The ability of the rectum to perceive the presence of stool leads to the rectoanal contractile reflex response, an essential mechanism for maintaining continence.
Pudendal neuropathy can diminish rectal sensation and lead to excessive accumulation of stool, causing fecal impaction, mega-rectum, and fecal overflow. The puborectalis muscle plays an integral role in maintaining the anorectal angle. Its nerve supply is independent of the sphincter, and its precise role in maintaining continence needs to be defined. Obstetric trauma, the most common cause of anal sphincter disruption, may involve the EAS, the IAS, and the pudendal nerves, singly or in combination.
It remains unclear why most women who sustain obstetric injury in their 20s or 30s typically do not present with fecal incontinence until their 50s. There is a strong need for prospective, long-term studies of sphincter function in nulliparous and multiparous women.
Fecal continence is maintained by the structural and functional integrity of the anorectal unit. Consequently, disruption of the normal anatomy or physiology of the anorectal unit leads to fecal incontinence. Fecal incontinence is often due to multiple pathogenic mechanisms and is rarely attributable to a single factor.
Structure and function of the anorectum
The rectum is a hollow muscular tube, 12 to 15 cm long, composed of a continuous layer of longitudinal muscle that interlaces with the underlying circular muscle. The anus is a muscular tube 2 to 4 cm long. At rest, it forms an angle of approximately 90 degrees with the axis of the rectum. During voluntary squeeze the angle becomes more acute, whereas during defecation, the angle becomes more obtuse
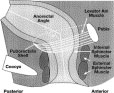 Figure 1. Structure of the anorectum. The internal anal sphincter muscle provides between 70% and 85% of resting sphincter pressure. The anorectal angle, approximately 90 degrees at rest, becomes more obtuse during defecation.
Figure 1. Structure of the anorectum. The internal anal sphincter muscle provides between 70% and 85% of resting sphincter pressure. The anorectal angle, approximately 90 degrees at rest, becomes more obtuse during defecation.
The anal sphincter
The anal sphincter consists of 2 muscular components: the internal anal sphincter (IAS), a 0.3-cm to 0.5-cm thick expansion of the circular smooth muscle layer of the rectum, and the external anal sphincter (EAS), a 0.6-cm to 1.0-cm thick expansion of the levator ani muscles. Morphologically, both sphincters are separate and heterogeneous. The IAS is a predominantly slow-twitch, fatigue-resistant smooth muscle. The IAS generates mechanical activity, with a frequency of 15 to 35 cycles per minute, and ultra-slow waves at 1.5 to 3 cycles per minute. The IAS contributes approximately 70% to 85% of the resting sphincter pressure, but only 40% after sudden distention of the rectum and 65% during constant rectal distention. Thus, the IAS is chiefly responsible for maintaining anal continence at rest.
The anus is normally closed by the tonic activity of the IAS. This barrier is reinforced during voluntary squeeze by the EAS. The anal mucosal folds, together with the expansive anal vascular cushions, provide a tight seal. These barriers are further augmented by the puborectalis muscle, which forms a flap-like valve that creates a forward pull and reinforces the anorectal angle.
Nerve structure and sensation
The anorectum is richly innervated by the sensory, motor, and autonomic nerves and by the enteric nervous system. The principal nerve is the pudendal nerve, which arises from the second, third, and fourth sacral nerves (S2, S3, S4) and innervates the EAS. The pudendal nerve is a mixed nerve that subserves both sensory and motor function. Pudendal nerve block creates a loss of sensation in the perianal and genital skin and weakness of the anal sphincter muscle, but does not affect rectal sensation. Pudendal nerve block also abolishes the rectoanal contractile reflexes, which suggests that pudendal neuropathy may affect the rectoanal contractile reflex response. The sensation of rectal distention is most likely transmitted along the S2, S3, and S4 parasympathetic nerves. These nerve fibers traverse along the pelvic splanchnic nerves and are independent of the pudendal nerve.
It is not completely understood how humans perceive stool contents in the anorectum. Earlier studies failed to demonstrate rectal sensory awareness. Subsequent studies confirmed that balloon distention is perceived in the rectum and that such perception plays a role in maintaining continence. Furthermore, sensory conditioning can improve both hyposensitivity and hypersensitivity of the rectum. Mechanical stimulation of the rectum can produce cerebral evoked responses, which confirms that the rectum is a sensory organ.
Although there are no organized nerve endings in the rectal mucosa or in the myenteric plexus, both myelinated and unmyelinated nerve fibers are present. These nerves most likely mediate the distention or stretch-induced sensory responses, as well as the viscero-visceral, the recto-anal inhibitory, and the recto-anal contractile reflexes. The sensation of rectal distention is most likely transmitted along the S2, S3, and S4 parasympathetic nerves. Rectal sensation and the ability to defecate can be abolished completely by resection of the nervi erigentes. If parasympathetic innervation is absent, rectal filling is only perceived as a vague sensation of discomfort. Even paraplegics or persons with sacral neuronal lesions may retain some degree of sensory function, but virtually no sensation is felt if lesions reach the higher spine. Thus, the sacral nerves are intimately involved with the maintenance of continence.
It has been suggested that bowel contents are periodically sensed by anorectal sampling, the process by which transient relaxation of the IAS allows the stool contents from the rectum to come into contact with specialized sensory organs, such as the Krause end-bulbs, Golgi-Mazzoni bodies and genital corpuscles, and the sparse Meissner’s corpuscles and Pacinian corpuscles in the upper anal canal. Specialized afferent nerves may exist that subserve sensations of touch, temperature, tension, and friction, but these are incompletely understood. Incontinent patients appear to sample rectal contents less frequently than continent subjects. The likely role of anal sensation is to facilitate discrimination between flatus and feces and the fine-tuning of the continence barrier, but its precise role needs to be characterized.
Rectal distention
Rectal distention is associated with a decrease in anal resting pressure known as the rectoanal inhibitory reflex. The amplitude and duration of this relaxation increases with the volume of rectal distention. This reflex is mediated by the myenteric plexus and is present in patients with transection of the hypogastric nerves and in patients with spinal cord lesions. It is absent after rectal transection but may recover. The arrival of flatus mimics sudden rectal distention and this is associated with a decrease in anal pressure.
Although the rectoanal inhibitory reflex may facilitate discharge of flatus, rectal distention is also associated with an anal contractile response, a subconscious reflex effort to prevent release of rectal contents, such as flatus. This contractile response involves contraction of the external anal sphincter - not the internal anal sphincter, as is true for the rectoanal inhibitory reflex - and it is mediated by the pelvic splanchnic and pudendal nerves. The amplitude and duration of the rectoanal contractile reflex also increases with rectal distention up to a maximum volume of 30 mL. Abrupt increases in intra-abdominal pressure, such as those caused by coughing or laughing, are associated with increases in anal sphincter pressure. This may be achieved through multiple mechanisms, including reflex contraction of the puborectalis.
Anal endovascular cushions
The blood-filled vascular tissue of the anal mucosa also plays an important role in producing a more perfect closure of the anus. An in vitro study showed that even during maximal involuntary contraction, the internal sphincter ring was unable to close the anal orifice completely and a gap of approximately 7 mm was left open. This gap was filled by the anal cushions, which may exert pressures of up to 9 mm Hg and thereby may contribute 10% to 20% of resting anal pressure.
Pathogenic mechanisms and etiology
Fecal incontinence occurs when one or more mechanisms that maintain continence are disrupted to an extent that other mechanisms are unable to compensate. Hence, fecal incontinence is often multifactorial. In a prospective study, 80% of patients with fecal incontinence had more than one pathogenic abnormality. Although the pathophysiological mechanisms often overlap, they may be categorized under the 4 subheadings shown in Table 1.
The probable causes of these pathophysiologies and the mechanisms by which they lead to fecal incontinence are also summarized in Table 1.
Table 1. Pathophysiological Mechanisms Leading to Fecal Incontinence
Structural abnormalities
Anal sphincter muscles
Disruption or weakness of the EAS muscle causes urge-related or diarrhea-associated fecal incontinence. In contrast, damage to the IAS muscle or the anal endovascular cushions may lead to a poor seal and an impaired sampling reflex. These changes may cause passive incontinence or fecal seepage, often under resting conditions. Both sphincters may be defective in many patients. The extent of muscle loss can influence the severity of incontinence.
The most common cause of anal sphincter disruption is obstetric trauma. However, it is unclear why most women who have sustained an obstetric injury in their 20s or 30s typically do not present with fecal incontinence until their 50s. The injury may involve the EAS, the IAS, the pudendal nerves, or a combination of these structures. In a prospective study, 35% of primiparous (normal antepartum) women showed evidence of anal sphincter disruption after vaginal delivery. Other important risk factors include forceps-assisted delivery, prolonged second stage of labor, large birth weight, and occipitoposterior presentations. Furthermore, perineal tears, even when carefully repaired, can be associated with incontinence, and patients may present several years after delivery.
Episiotomy is believed to be a risk factor for anal sphincter disruption. In one study, medial episiotomy was associated with a 9-fold higher risk for anal sphincter dysfunction. However, in a large, 30-year retrospective cohort study, the prevalence of frequent fecal incontinence was 6.9% for women whose index delivery was complicated by anal sphincter disruption, 18% for women with episiotomy, and 0% for women who had cesarean section; bothersome incontinence was experienced by 27.6%, 25.8%, and 15.2% of the respective groups. This study suggests that regardless of the type of delivery, fecal or flatus incontinence occurs in a surprisingly large percentage of middle-aged women. This raises the issue of whether age-related changes that affect the pelvic floor are a predisposing comorbid problem in the pathogenesis of fecal incontinence.
Whether anal sphincter pressures change with aging is debatable. In both men and women more than 70 years of age, there was a 30% to 40% decrease in sphincter pressures compared with patients younger than 30 years of age. In another study, elderly subjects were found to have lower sphincter pressures, but many were taking medications that may have affected muscle function. Other trials that examined anal pressures have reported only insignificant decreases with age. However, in all age groups squeeze pressure has been shown to be significantly lower in women than in men, with an apparently rapid decrease after menopause. Recently, estrogen receptors have been identified in the human striated anal sphincter. In experimental studies of adult rats, ovariectomy led to atrophy of the striated anal sphincter muscle, which suggests that the strength and vigor of the pelvic floor muscles is influenced by hormones. Pudendal nerve terminal motor latency (PNTML) is prolonged in older women, and there is excessive pelvic floor descent on straining. These mechanisms may lead to progressive damage to the striated anal sphincter muscle due to repeated stretch injury during straining. An anal endosonography study also showed that aging was associated with an increase in the thickness and echogenicity of the internal sphincter muscle.
Other causes of anatomic disruption include anorectal surgery for hemorrhoids, fistula, and fissures. Anal dilation or lateral sphincterotomy may result in permanent incontinence due to fragmentation of the anal sphincter apparatus. Contrary to the belief of many surgeons, hemorrhoidectomy can cause incontinence by inadvertently damaging the IAS or through the loss of endovascular cushions. Accidental perineal trauma or a pelvic fracture may also cause direct sphincter trauma leading to fecal incontinence. Interestingly, a study of homosexual men showed that although the anal resting pressure of the subjects was lower and the anal sphincters were thinner than those of controls, there was no evidence of sphincter injury from anoreceptive intercourse. These results suggest that anal intercourse may not cause sphincter trauma, at least in men. Finally, in the absence of traumatic structural defects, internal sphincter dysfunction may also occur because of myopathy, internal sphincter degeneration, or as a complication of radiotherapy.
Puborectalis muscle
Sir Allan Parks believed that the pressure exerted by the anterior rectal wall, together with the puborectalis muscle, was fundamental to maintaining continence by forming a flap valve mechanism. This concept was disputed in another study that imaged the rectum radiologically while simultaneously measuring rectal and anal canal pressures and anal electromyogram (EMG) activity during defecation maneuvers. The authors concluded that continence was maintained primarily by increased activity of the EAS muscle and the puborectalis muscle and that rectal pressures were consistently lower than those generated within the anal canal. Similar observations were made by another group. Also, after successful sphincter repair, continence was associated with higher sphincter pressures and not with an altered anorectal angle. These findings suggest that an obtuse anorectal angle may represent an epiphenomenon in patients with incontinence.
The nerve supply for the upper portion of the puborectalis muscle arises from direct branches of the anterior S3 and S4 rather than traveling with the pudendal nerve. Thus, the puborectalis muscle and the EAS have separate neurological innervation. Consequently, pudendal blockage does not abolish voluntary contraction of the pelvic floor but completely abolishes EAS function. It has been suggested that continence can be preserved following division of the EAS and IAS provided the puborectalis muscle is intact. Moreover, division of the puborectalis muscle posteriorly does not produce incontinence as long as anal sphincter pressures are normal. A recent study, using a novel perineal dynamometer, showed that the levator ani muscle may play an important and integral role in preserving continence, along with the anal sphincter.
Neuropathy
An intact innervation of the pelvic floor is essential for maintaining continence. Sphincter degeneration secondary to pudendal neuropathy and obstetric trauma may cause fecal incontinence in women. The neuropathic injury is often sustained during childbirth, probably due to stretching of the nerves during elongation of the birth canal or through direct trauma during the passage of the fetal head. The nerve damage is more likely to occur when the fetal head is large, when the second stage of labor is prolonged, and when forceps are applied, especially high forceps delivery or if there is prolonged labor. Damage to the innervation of the pelvic floor musculature is usually asymmetrical. Subsequent vaginal deliveries may further damage the pudendal nerves. In another study of women who sustained obstetric sphincter injury, the only risk factor associated with the development of fecal incontinence was prolonged PNTML.
Autonomic neuropathy
The role of extrinsic autonomic innervation is somewhat controversial. Animal studies have shown that the pelvic nerves convey relaxatory fibers to the rectum. Consequently, these nerves may play a role in accommodating and storing feces and gas. Damage to the pelvic nerves may lead to impaired accommodation and rapid transit through the rectosigmoid region, overwhelming the continence barrier mechanisms. Sympathetic efferent activity, as studied by stimulating the presacral sympathetic nerves, tends to relax the IAS, whereas parasympathetic stimulation may cause contraction of the anal sphincter. The upper motor neurons for voluntary sphincter muscle lie close to those innervating the lower limb muscles in the parasagital motor cortex adjacent to the sensory representation of the genitalia and perineum in the sensory cortex. Consequently, damage to the motor cortex from central nervous system (CNS) lesions may lead to incontinence. In some patients with neurogenic incontinence, there is damage to both the sensory and motor nerve fibers, resulting in sensory impairment. This damage can impair conscious awareness of rectal filling as well as the associated reflex responses in the striated pelvic floor sphincter muscles.
Approximately 10% of patients with fecal incontinence may have lesions more proximal than the intrapelvic or perianal nerves. The primary abnormality in these patients is cauda equina nerve injury, which may be occult and not evident through clinical evaluation. These patients have a prolongation of nerve conduction along the cauda equina nerve roots without an abnormality in PNTML. In a minority of patients, however, there is a combination of peripheral and central lesions. Other disorders such as multiple sclerosis, diabetes, and demyelination injury (or toxic neuropathy from alcohol or traumatic neuropathy) may also lead to incontinence.
Rectal accommodation and reservoir function
The rectum is a compliant reservoir that stores stool until social conditions are conducive for its evacuation. If rectal wall compliance is impaired, a small volume of stool material can generate high intrarectal pressure that can overwhelm anal resistance and cause incontinence. Etiologies include radiation proctitis, ulcerative colitis, or Crohn’s disease, infiltration of the rectum by tumor, or following radical hysterectomy. Similarly, rectal surgery, and particularly pouch surgery and spinal cord injury, may be associated with loss of rectal compliance.
Functional mechanisms
Anorectal sensation
An intact sensation not only provides a warning of imminent defecation, but also helps to discriminate between formed stool, liquid feces, or flatus. Elderly persons, physically and mentally challenged individuals, and children with fecal incontinence often show blunted rectal sensation. Impaired rectal sensation may lead to excessive accumulation of stool, causing fecal impaction, mega-rectum (extreme dilation of the rectum), and fecal overflow. Causes of impaired sensation include neurological damage such as multiple sclerosis, diabetes mellitus, or spinal cord injury. Less well known is the fact that analgesics (particularly opiates) and antidepressants may also impair rectal sensation and produce fecal incontinence. The importance of the rectum in preserving continence has been demonstrated conclusively through surgical studies in which preservation of the distal 6 to 8 cm of the rectum, along with its parasympathetic nerve supply, helped subjects avoid incontinence. In contrast, both rectal sensation and the ability to defecate can be abolished completely by resection of the nervi erigentes.
An intact sampling reflex allows the individual to choose whether to discharge or retain rectal contents. Conversely, an impaired sampling reflex may predispose a subject to incontinence. However, the role of the sampling reflex in maintaining continence remains unclear. In children who have had colonic pull-through surgery, some degree of sensory discrimination is preserved. Because the anal mucosal sensory zone is absent, it has been suggested that sensory receptors, possibly located in the puborectalis muscle, may play a role in facilitating sensory discrimination. Also, traction of this muscle is a more potent stimulus for triggering both defecation and a sensation of rectal distention. Because abolition of anal sensation by the topical application of 5% lidocaine did not reduce resting sphincter pressure - although it partially affected voluntary squeeze pressure and did not affect the ability to retain saline infused into the rectum - the role of anal sensation in maintaining fecal continence has been questioned.
Dyssynergic defecation and incomplete stool evacuation
In some patients, particularly the elderly, prolonged retention of stool in the rectum or incomplete evacuation may lead to seepage of stool or staining of undergarments. A majority of these patients show obstructive or dyssynergic defecation, and many of them also exhibit impaired rectal sensation, whereby anal sphincter and pudendal nerve function is intact, but the ability to evacuate a stimulated stool is impaired. Similarly, in the elderly and in children with functional incontinence, the prolonged retention of stool in the rectum can lead to fecal impaction. Fecal impaction may also cause prolonged relaxation of IAS tone, which allows liquid stool to flow around impacted stool and to escape through the anal canal.
Descending perineum syndrome
In women with long-standing constipation and a history of excessive straining for many years (perhaps even without a childbirth), excessive straining may lead to progressive denervation of the pelvic floor muscles. Most of these patients demonstrate excessive perineal descent and sphincter weakness, which may lead to rectal prolapse; however, fecal incontinence is not an inevitable consequence. Whether or not incontinence develops will depend on the state of the pelvic floor and the strength of the sphincter muscles.
Stool characteristics
The consistency, volume, and frequency of stool and the presence or absence of irritants in stool may also play a role in the pathogenesis of incontinence In the presence of large-volume liquid stools, which often transit the hind-gut rapidly, continence can only be maintained through intact sensation and a strong sphincteric barrier. Similarly, in patients with bile salt malabsorption, or lactose or fructose intolerance or rapid dumping of osmotic material into the colon, the colonic transit is too rapid for both gaseous and stool contents and can overwhelm the continence mechanisms.
Miscellaneous
A variety of medical conditions and disabilities may predispose to fecal incontinence, particularly in the elderly. Immobility and lack of access to toileting facilities are primary causes of fecal incontinence in this population. Several medications may inhibit sphincter tone - for example, anticholinergics, some of which are used to treat urinary incontinence and detrusor muscle instability, include tolterodine tartarate (Detrol), Pharmacia, Kalamazoo, MI; oxybutynin (Ditropan), Alza Pharmaceuticals, Palo Alto, CA; and muscle relaxants such as baclofen (Lioresal), Novartis Pharmaceuticals, Summit, NJ; cyclobenzaprine (Flexeril), Merck Pharmaceuticals, Fort Washington, PA; and antispasmodics. In contrast, stimulants such as caffeinated products, fiber supplements, or laxatives may cause diarrhea.
References
1. S.S.C. Rao and R.S. Patel, How useful are manometric tests of anorectal function in the management of defecation disorders?. Am J Gastroenterol 92 (1997), pp. 469–475.
2. S.S.C. Rao, Fecal incontinence. Clin Perspect Gastroenterol 2 (1999), pp. 277–288.
3. K.E. Matzel, R.A. Schmidt and E.A. Tanagho, Neuroanatomy of the striated muscular anal continence mechanism. Implications for the use of neurostimulation. Dis Colon Rectum 33 (1990), pp. 666–673.
4. S. Salmons and G. Vrbova, The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol 201 (1969), pp. 535–549.
5. M.A. Johnson, J. Polgar, D. Weightman and D. Appleton, Data on the distribution of the fiber types in thirty-six human muscles: an autopsy study. J Neurol Sci 18 (1973), pp. 111–129.
6. R. Kerremans, Electrical activity and motility of the internal anal sphincter. Acta Gastroenterol Belg 31 (1968), pp. 465–482.
7. W.J. Wankling, B.H. Brown, C.D. Collins and H.L. Duthie, Basal electrical activity in the anal canal in man. Gut 9 (1968), pp. 457–460.
8. B. Frenckner and C. Von Euler, Influence of pudendal block on the function of the anal sphincter. Gut 16 (1975), pp. 482–489.
9. C.P. Gibbons, J.J. Bannister, E.A. Trowbridge and N.W. Read, An analysis of anal sphincter pressure and anal compliance in normal subjects. Int J Colorectal Dis 1 (1986), pp. 231–237.
10. C.P. Gibbons, E.A. Trowbridge, J.J. Bannister and N.W. Read, Role of anal cushions in maintaining continence. Lancet 1 (1986), pp. 886–888.
11. A.G. Parks, N.H. Porter and J.D. Hardcastle, The syndrome of descending perineum. Proc R Soc Med 59 (1966), pp. 477–482.
12. B. Gunterberg, J. Kewenter, I. Petersen and B. Stener, Anorectal function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Br J Surg 63 (1976), pp. 546–554.
13. H.L. Duthie and F.W. Gaims, Sensory nerve-endings and sensation in the anal region of man. Br J Surg 47 (1960), pp. 585–595.
14. J.C. Goligher and E.R.S. Hughes, The sensibility of colon and rectum. Lancet 1 (1951), pp. 543–547.
15. M.G. Read and N.W. Read, Role of anorectal sensation in preserving continence. Gut 23 (1982), pp. 345–347.
16. W.M. Sun, N.W. Read and T.C. Donnelly, Anorectal function in incontinent patients with spinal disease. Gastroenterology 99 (1990), pp. 1372–1379.
Abbreviations: CNS, central nervous system; EAS, external anal sphincter; EMG, electromyogram; IAS, internal anal sphincter; PNTML, pudendal nerve terminal motor latency
Satish S. C. Rao
Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA