Ovarian Development during Puberty and Adolescence
Before the increasingly common use of ultrasound evaluation of the ovaries, anatomical and histological descriptions gave us a dynamic image of the developing ovary documenting that during childhood the ovary is never at rest and follicles grow and degenerate continuously. Peters et al. identified three steps of ovarian growth: the quiescent ovary, with small resting follicles and occasional preantral follicles; the ovary in early growth, with small follicles, preantral follicles and occasional antral follicles not larger than 0.5mm; and the actively growing ovary that is more and more frequent after the age of 6 when antral follicles, which are usually well distended, become increasingly numerous and large. These follicles have been called ‘cystic’ but they are normal follicles, some of them healthy, others in degeneration.
The ovarian size increases throughout childhood in relation to a gradual increase in the number as well as in the size of the antral follicles and a progressive increase of the stroma derived from follicle atresia. Besides multiple follicle growth and atresia, partial luteinization of the theca interna and fibrosis of the cortex are common features in the normal process of follicular development throughout infancy and childhood, so that early pubertal ovaries have been described that are indistinguishable from those of the polycystic ovary syndrome.
The advent of ultrasound has enabled clinicians to easily examine ovaries of a large number of girls and, moreover, to follow their development (fig. 4).
Antral follicles or microcysts not exceeding 9 mm, as defined by ultrasound, start to increase around the age of 9 with the appearance of fluid areas >9mm after the age of 11.
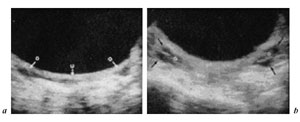
Fig. 4. Ultrasound ovarian appearance before puberty (a) and after puberty onset (b) [from 1].
In the years immediately before menarche, the cystic structure becomes prevalent. The increase in cystic structure coincides with the beginning of the gonadotropin rise which seems to play a central role in the last stages of follicular development. At this age gonadotropin secretion changes its quality, enhancing its pulsatile characteristics, both episodic and circadian, and sending a faster and more fluctuating signal to the ovaries which accelerate the turnover of follicular development. The follicles increase in size and steroid production.
Figure 5 shows an early pubertal nocturnal rise of LH, with levels remaining very low during the day. However, only a few nocturnal pulses are sufficient to promote the progression of some follicles in the still small ovaries. A higher number of LH pulses can occasionally be present also during the day in premenarcheal girls at mid and late puberty when ovaries show a higher volume and number of follicles compared to the previous stage (fig. 5 - Gonadotropin profiles and related sonographic ovarian appearance at (a) early, (b) mid and (c) late puberty.). In fact there is a positive correlation between Tanner stages and ovarian volume(fig. 6)
.The functional result of these integrated movements is the increase in steroidogenesis. Indeed, there is a positive correlation between ovarian volume, estradiol (E2) and testosterone (T). In the same subject we can find greater or lesser follicular activity at different times in this period of life. Therefore, before menarche, multifollicular ovaries mean more and more activated ovaries but sometimes overactivated ovaries.
Just before menarche, puberty is characterized by the interaction of two evolving systems: the increasing gonadotropins and the actively growing ovary.
For correct reproductive development, the two systems should interact in the most appropriate way and time and after puberty the dominant follicle growth should take over, with a decrease in the number of developing follicles.
Any derangement, i.e., an abnormal gonadotropin signal or an excessively prolonged process, may potentially lead to pathology. According to the number of developing follicles the sonographic appearance of the ovaries may be homogeneous, when fewer than four cystic areas are present, and multifollicular, when four or more fluid areas are imaged by ultrasound. It must be stressed, however, that this is an oversimplification since a wide spectrum of images, with a variable number of developing follicles, may be found.
Figure 7
shows that girls with regular cycles have normal ovarian volume and a homogeneous appearance. Girls with irregular cycles, however, have a mean volume significantly higher than those of regular ovulatory adolescents and those of adults. The postpubertal developing ovary is then mostly enlarged and multifollicular. What is the differentiating element with polycystic ovaries (PCO)? Many authors have tried to classify the ‘jungle’ of ovaries with ‘multiple echo-free areas’ or ‘fluid images’, according to the number, size and distribution of cystic areas, the amount of stroma, the ovarian volume, the uterine-ovarian ratio, and the morphofunctional responses to induction of ovulation.An appropriate classification might be the one outlined in table 1 where multifollicular ovaries have more than 5 cystic areas spread throughout the stroma (
fig. 8) and polycystic ovaries have more than 10 cysts arrayed peripherically (fig. 9
).Fig. 8. Sonographic aspect of a multifollicular ovary.
Fig. 9. Sonographic aspect of a polycystic ovary.Adolescent ovarian development passes through different sonographic stages to lead to the normal adult ovary. This evolution is driven by the increasing number and regularity of ovulations. The more ovulations, the lower the ovarian volume and the number of follicles.
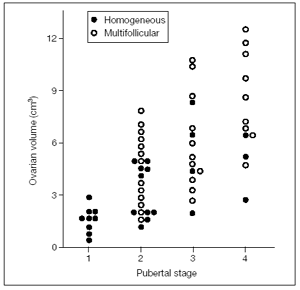
Fig. 10. Ovarian volumes at various frequencies of ovulation. A = Anovulation [from 8].
The time necessary to ‘learn’ how to ovulate seems crucial for the development of a normal ovarian structure. There seems to exist a sort of temporal window in which the ovaries are still plastic and can undergo morphological changes.
Outside of this framework, the ovary has great difficulty moving beyond the adolescent structure. Years of anovulation and particularly of increased LH secretion mark the ovary irreversibly, increasing the stroma and turning the multifollicular aspects into the polycystic structure.
The duration of anovulation is probably the key factor responsible for the onset of the persistence and worsening of the polycystic structure.
A prospective longitudinal study documented that in the years after the menarche, the ovarian volume changes from normal to enlarged in 30% of the adolescent population (
fig. 11 - Longitudinal examination of the ovaries. Distribution of the subjects (n = 46) with normal ovarian volume during the first control (left) and outcome of the ovarian volume and morphology as found during last control (right).
). The postmenarcheal period is frequently the starting point for the polycystic transformation of the ovaries.By contrast, in some subjects with enlarged multifollicular and polycystic ovaries (22%), ovarian volume can become normal (
fig. 12
). It is well known that multifollicular morphology of the ovaries may revert to normal when ovulation is achieved. On the other hand, the possibility of achieving a normal adult echographic image with a homogeneous structure of the ovaries during the postmenarcheal period was not observed for the polycystic ovaries. A shift from polycystic to multifollicular appearance was detected in 2 cases only; this is sufficient to confirm that the transition from one structure to the other may occur. A cross-selectional study documented in 139 adolescents a relatively high frequency of ovarian cysts (12%). Most of them were simplefollicular cysts (fig. 13) and disappeared spontaneously. One cyst was a benign teratoma (fig. 14) and one was an endometrioma (fig. 15) and required surgical intervention. Another possible ovarian pathology is cystoadenoma (
fig. 16
).
These data suggest that serial ultrasound evaluations are indicated in adolescence to monitor the correct ovarian development and to prevent or early diagnose pathology.
References
- Orsini LR, Salardi S, Pilu G, Bovicelli L, Cacciari E: Pelvic organs in premenarcheal girls: Realtime ultrasonography. Radiology 1984;153:113.
- Porcu E, Venturoli S, Fabbri R, Orsini LF, Sganga E, Brondelli L, Paradisi R, Flamingni C: Uterine development and endocrine relationships after menarche. Am J Obstet Gynecol 1989;161: 174–177.
- Peters H, Byskov AG, Himelstein-Braw R, Faber M: Follicular growth: The basic event in the mouse and human ovary. J Reprod Fertil 1975;45:559.
- Venturoli S, Porcu E, Fabbri R, Paradisi R, Orsini LF, Flamigni C: Ovaries and menstrual cycles in adolescence. Gynecol Obstet Invest 1984;17:219–222.
- Tucker M, Adams J, Mason WP, Jacobs HS: Infertility, megalocystic and polycystic ovaries: Differential response to LHRH therapy. Ups J Med Sci 1984;89:43.
- Adams J, Polson DW, Abdulwahid N, Morris DV, Franks S, Mason HD, Tucker M, Price J, Jacobs HS: Multifollicular ovaries: Clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 1985;ii:1375.
- Treasure JL, King EA, Gordon PAL, Wheeler M, Russell GEM: Cystic ovaries: A phase of anorexia nervosa. Lancet 1985;ii:1379.
- Venturoli S, Fabbri R, Porcu E, Paradisi R, Orsini LF, Brondelli L, Ruggeri S, Flamigni C: Endocrine and ovarian parameters at various frequencies of ovulation in adolescents. Arch Gynecol Obstet 1989;246:107.
- Venturoli S, Porcu E, Fabbri R, Pluchinotta V, Ruggeri S, Macelli S, Paradisi R, Flamigni C: Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–980.
- Porcu E, Venturoli S, Magrini O, Bolzani R, Gabbi D, Paradisi R, Fabbri R, Flamigni C: Circadian variations of luteinizing hormone can have two different profiles in adolescent anovulation. J Clin Endocrinol Metab 1987;65:488.
- Porcu E, Venturoli S, Flamigni C: Relations between puberty and the onset of the polycystic ovary syndrome; in Major Advances in Human Female Reproduction. New York, Raven Press, 1991, vol 73, p 45.
- Porcu E, Venturoli S, Longhi M, Fabbri R, Paradisi R, Flamigni C: Chronobiologic evolution of luteinizing hormone secretion in adolescence: Development patterns and speculation on the onset of the polycystic ovary syndrome. Fertil Steril 1997;67:5.
- Porcu E, Venturoli S, Dal Prato L, Fabbri R, Paradisi R, Flamigni C: Frequency and treatment of ovarian cysts in adolescence. Arch Gynecol Obstet 1994;255:69–72.
Revision date: July 7, 2011
Last revised: by Jorge P. Ribeiro, MD